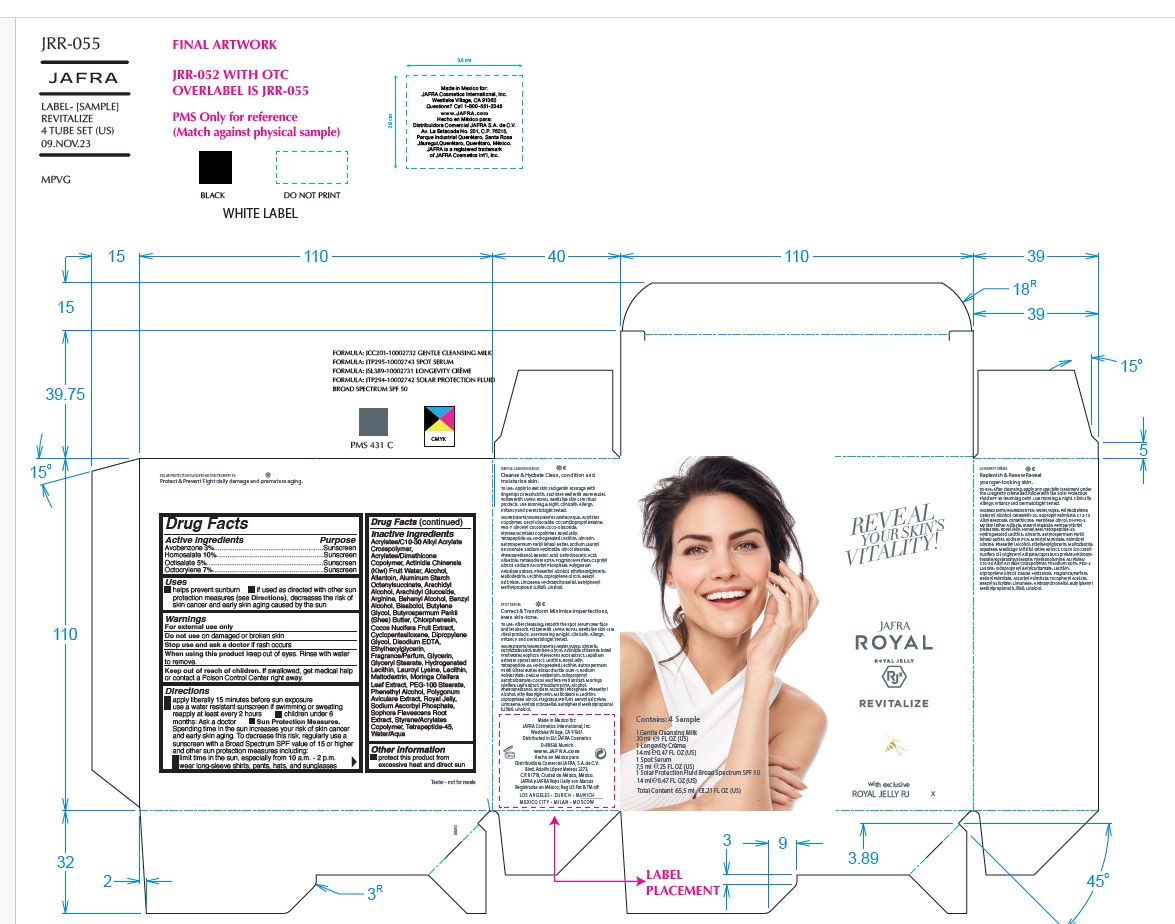
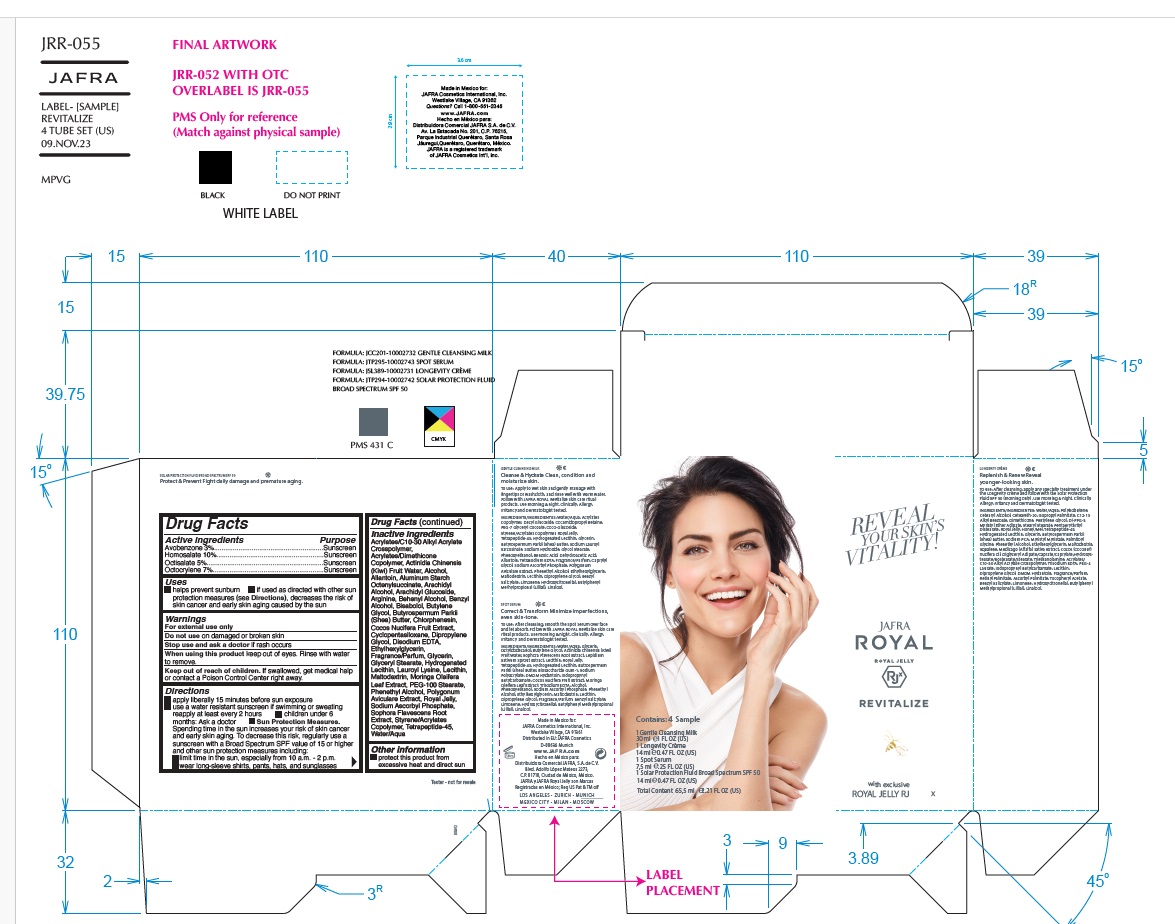
Label: JAFRA ROYAL DEFY SAMPLE KIT- avobenzone, homosalate, octisalate, octocrylene kit
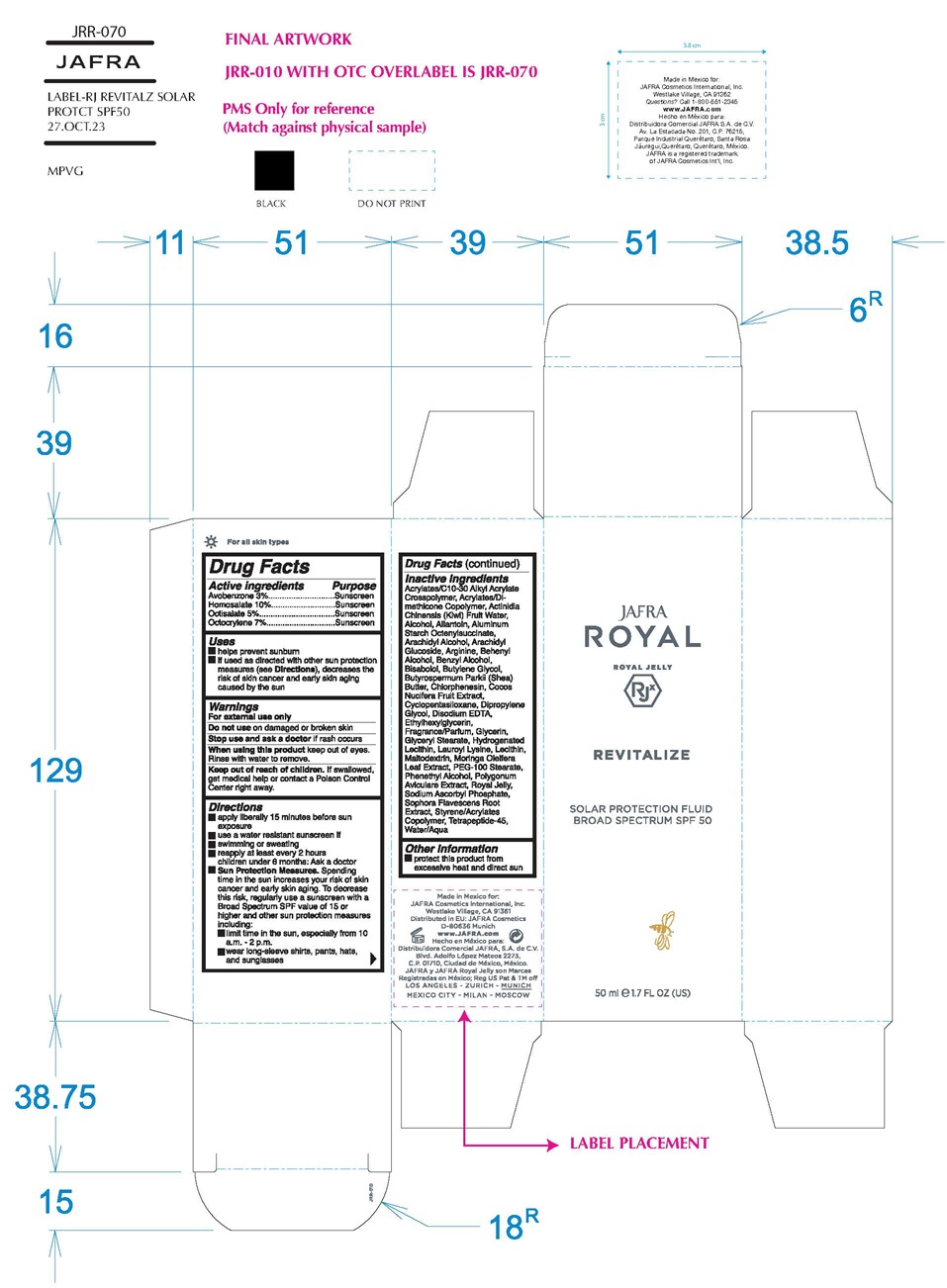
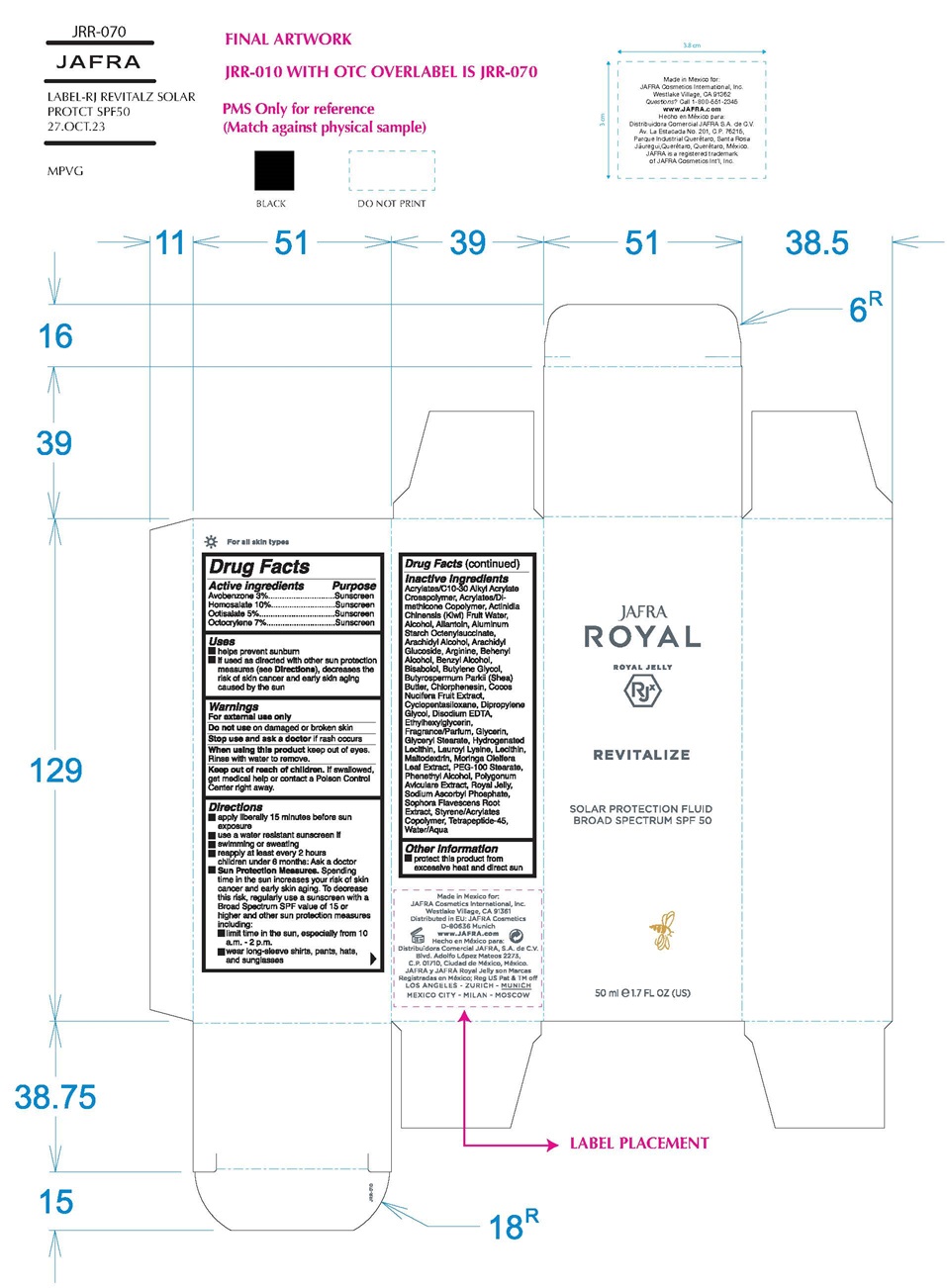
REVITALIZE SOLAR PROTECTION FLUID BROAD SPECTRUM SPF 50- avobenzone, homosalate, octisalate, octocrylene cream
JAFRA ROYAL DEFY TRAVEL KIT- avobenzone, homosalate, octisalate, octocrylene kit
-
NDC Code(s):
68828-511-01,
68828-512-01,
68828-513-01,
68828-514-01, view more68828-515-01
- Packager: Jafra cosmetics International
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- apply generously and evenly 15 minutes before sun exposure
- use a water-resistant sunscreen if swimming or sweating
- reapply at least every 2 hours.
- children under 6 months of age: Ask a doctor
- Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad-Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit your time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
-
Inactive ingredients
Water/Aqua, Styrene/Acrylates Copolymer, Aluminum Starch Octenylsuccinate, Ethylhexylglycerin, Benzyl Alcohol, Glyceryl Stearate, PEG-100 Stearate, Arachidyl Alcohol, Bisabolol, Behenyl Alcohol, Cyclopentasiloxane, Lauroyl Lysine, Arachidyl Glucoside, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Allantoin, Arginine, Chlorphenesin, Disodium EDTA, Acrylates/Dimethicone Copolymer, Royal Jelly Powder, Glycerin, Butyrospermum Parkii (Shea) Butter, Dipropylene Glycol, Sodium Ascorbyl Phosphate, Lecithin, Hydrogenated Lecithin, Royal Jelly, Polygonum Aviculare Extract, Phenethyl Alcohol, Cocos Nucifera (Coconut) Fruit Extract, Butylene Glycol, Actinidia Chinensis (Kiwi) Fruit Water, Moringa Oleifera Leaf Extract, Maltodextrin, Tetrapeptide-45, Alcohol, Sophora Flavescens Root Extract, Fragrance/Parfum, Benzyl Salicylate, Butylphenyl Methylpropional, Hydroxycitronellal, Limonene, Linalool
- Other information
- SPL UNCLASSIFIED SECTION
- Product label
-
INGREDIENTS AND APPEARANCE
JAFRA ROYAL DEFY SAMPLE KIT
avobenzone, homosalate, octisalate, octocrylene kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-511 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-511-01 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 08/22/2022 12/31/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 7.5 mL Part 2 1 TUBE 7.5 mL Part 3 1 TUBE 7.5 mL Part 4 1 TUBE 7.5 mL Part 1 of 4 GENTLE CLEANSING MILK
other skin care preparations, leave-on [skin care preparations (creams, lotions, powder, and sprays)] creamProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR PEG-7 GLYCERYL COCOATE (UNII: VNX7251543) INGR COCO GLUCOSIDE (UNII: ICS790225B) INGR DECYL GLUCOSIDE (UNII: Z17H97EA6Y) INGR STYRENE/ACRYLAMIDE COPOLYMER (500000 MW) (UNII: 5Z4DPO246A) INGR SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) INGR GLYCOL STEARATE (UNII: 0324G66D0E) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR ALLANTOIN (UNII: 344S277G0Z) INGR EDETATE SODIUM (UNII: MP1J8420LU) INGR BENZOIC ACID (UNII: 8SKN0B0MIM) INGR DEHYDROACETIC ACID (UNII: 2KAG279R6R) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR POLYGONUM AVICULARE TOP (UNII: ZCD6009IUF) INGR ROYAL JELLY (UNII: L497I37F0C) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR DIPROPYLENE GLYCOL (UNII: E107L85C40) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INGR PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR MALTODEXTRIN (UNII: 7CVR7L4A2D) INGR BENZYL SALICYLATE (UNII: WAO5MNK9TU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 7.5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 08/22/2022 Part 2 of 4 SPOT SERUM
other skin care preparations, leave-on [skin care preparations (creams, lotions, powder, and sprays)] creamProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR OCTYLDODECANOL (UNII: 461N1O614Y) INGR BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) INGR SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR KIWI FRUIT OIL (UNII: 66086CWP3Q) INGR DMDM HYDANTOIN (UNII: BYR0546TOW) INGR COCONUT (UNII: 3RT3536DHY) INGR EDETATE TRISODIUM (UNII: 420IP921MB) INGR SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) INGR MORINGA OLEIFERA LEAF (UNII: 4WET1AWO9B) INGR ALCOHOL (UNII: 3K9958V90M) INGR SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) INGR DIPROPYLENE GLYCOL (UNII: E107L85C40) INGR GARDEN CRESS SPROUT (UNII: PWQ18YNR62) INGR HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INGR PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) INGR ROYAL JELLY (UNII: L497I37F0C) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR MALTODEXTRIN (UNII: 7CVR7L4A2D) INGR BENZYL SALICYLATE (UNII: WAO5MNK9TU) INGR BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) INGR HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 7.5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 08/22/2022 Part 3 of 4 LONGEVITY CREME
other skin care preparations, leave-on [skin care preparations (creams, lotions, powder, and sprays)] creamProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR POLYISOBUTYLENE (800000 MW) (UNII: Y132ZOQ9H7) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INGR POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) INGR ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) INGR ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) INGR DIMETHICONE (UNII: 92RU3N3Y1O) INGR PENTYLENE GLYCOL (UNII: 50C1307PZG) INGR DI-PPG-3 MYRISTYL ETHER ADIPATE (UNII: T32481VTXW) INGR STEARYL STEARATE (UNII: 5WX2EGD0DK) INGR PENTAERYTHRITYL DISTEARATE (UNII: 697WOT8HNB) INGR ROYAL JELLY (UNII: L497I37F0C) INGR HONEY (UNII: Y9H1V576FH) INGR HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) INGR MYRISTYL MYRISTATE (UNII: 4042ZC00DY) INGR PALMITOYL GLYCINE (UNII: M6V3RIU5KI) INGR PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR MALTODEXTRIN (UNII: 7CVR7L4A2D) INGR SQUALENE (UNII: 7QWM220FJH) INGR COCONUT OIL (UNII: Q9L0O73W7L) INGR TROLAMINE (UNII: 9O3K93S3TK) INGR CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) INGR EDETATE TRISODIUM (UNII: 420IP921MB) INGR PEG-4 LAURATE (UNII: AYF4VM3N1Z) INGR IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR DIPROPYLENE GLYCOL (UNII: E107L85C40) INGR DMDM HYDANTOIN (UNII: BYR0546TOW) INGR VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) INGR ASCORBYL PALMITATE (UNII: QN83US2B0N) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR BENZYL SALICYLATE (UNII: WAO5MNK9TU) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) INGR HYDROXYCITRONELLOL (UNII: R0B4U2I48W) INGR BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) INGR LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 7.5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 08/26/2022 Part 4 of 4 REVITALIZE SOLAR PROTECTION FLUID BROAD SPECTRUM SPF 50
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Item Code (Source) NDC:68828-512 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) KIWI FRUIT OIL (UNII: 66086CWP3Q) ALCOHOL (UNII: 3K9958V90M) ALLANTOIN (UNII: 344S277G0Z) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) ARGININE (UNII: 94ZLA3W45F) DOCOSANOL (UNII: 9G1OE216XY) BENZYL ALCOHOL (UNII: LKG8494WBH) LEVOMENOL (UNII: 24WE03BX2T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SHEA BUTTER (UNII: K49155WL9Y) CHLORPHENESIN (UNII: I670DAL4SZ) COCONUT (UNII: 3RT3536DHY) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIPROPYLENE GLYCOL (UNII: E107L85C40) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) LAUROYL LYSINE (UNII: 113171Q70B) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MALTODEXTRIN (UNII: 7CVR7L4A2D) MORINGA OLEIFERA LEAF (UNII: 4WET1AWO9B) PEG-100 STEARATE (UNII: YD01N1999R) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) POLYGONUM AVICULARE TOP (UNII: ZCD6009IUF) ROYAL JELLY (UNII: L497I37F0C) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) STYRENE/ACRYLAMIDE COPOLYMER (500000 MW) (UNII: 5Z4DPO246A) WATER (UNII: 059QF0KO0R) BENZYL SALICYLATE (UNII: WAO5MNK9TU) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-512-01 7.5 mL in 1 TUBE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/22/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/22/2022 REVITALIZE SOLAR PROTECTION FLUID BROAD SPECTRUM SPF 50
avobenzone, homosalate, octisalate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-515 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) KIWI FRUIT OIL (UNII: 66086CWP3Q) ALCOHOL (UNII: 3K9958V90M) ALLANTOIN (UNII: 344S277G0Z) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) ARGININE (UNII: 94ZLA3W45F) DOCOSANOL (UNII: 9G1OE216XY) BENZYL ALCOHOL (UNII: LKG8494WBH) LEVOMENOL (UNII: 24WE03BX2T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SHEA BUTTER (UNII: K49155WL9Y) CHLORPHENESIN (UNII: I670DAL4SZ) COCONUT (UNII: 3RT3536DHY) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIPROPYLENE GLYCOL (UNII: E107L85C40) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) LAUROYL LYSINE (UNII: 113171Q70B) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MALTODEXTRIN (UNII: 7CVR7L4A2D) MORINGA OLEIFERA LEAF (UNII: 4WET1AWO9B) PEG-100 STEARATE (UNII: YD01N1999R) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) POLYGONUM AVICULARE TOP (UNII: ZCD6009IUF) ROYAL JELLY (UNII: L497I37F0C) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) STYRENE/ACRYLAMIDE COPOLYMER (500000 MW) (UNII: 5Z4DPO246A) WATER (UNII: 059QF0KO0R) BENZYL SALICYLATE (UNII: WAO5MNK9TU) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-515-01 1 in 1 CARTON 08/22/2022 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/22/2022 JAFRA ROYAL DEFY TRAVEL KIT
avobenzone, homosalate, octisalate, octocrylene kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-513 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-513-01 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 08/22/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 30 mL Part 2 1 TUBE 7.5 mL Part 3 1 TUBE 14 mL Part 4 1 TUBE 14 mL Part 1 of 4 GENTLE CLEANSING MILK
other skin care preparations, leave-on [skin care preparations (creams, lotions, powder, and sprays)]Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR PEG-7 GLYCERYL COCOATE (UNII: VNX7251543) INGR COCO GLUCOSIDE (UNII: ICS790225B) INGR DECYL GLUCOSIDE (UNII: Z17H97EA6Y) INGR STYRENE/ACRYLAMIDE COPOLYMER (500000 MW) (UNII: 5Z4DPO246A) INGR SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) INGR GLYCOL STEARATE (UNII: 0324G66D0E) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR ALLANTOIN (UNII: 344S277G0Z) INGR EDETATE SODIUM (UNII: MP1J8420LU) INGR BENZOIC ACID (UNII: 8SKN0B0MIM) INGR DEHYDROACETIC ACID (UNII: 2KAG279R6R) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR POLYGONUM AVICULARE TOP (UNII: ZCD6009IUF) INGR ROYAL JELLY (UNII: L497I37F0C) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR DIPROPYLENE GLYCOL (UNII: E107L85C40) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INGR PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR MALTODEXTRIN (UNII: 7CVR7L4A2D) INGR BENZYL SALICYLATE (UNII: WAO5MNK9TU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 08/22/2022 Part 2 of 4 SPOT SERUM
other skin care preparations, leave-on [skin care preparations (creams, lotions, powder, and sprays)]Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR OCTYLDODECANOL (UNII: 461N1O614Y) INGR BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) INGR SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR ROYAL JELLY (UNII: L497I37F0C) INGR KIWI FRUIT OIL (UNII: 66086CWP3Q) INGR DMDM HYDANTOIN (UNII: BYR0546TOW) INGR COCONUT (UNII: 3RT3536DHY) INGR EDETATE TRISODIUM (UNII: 420IP921MB) INGR SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) INGR MORINGA OLEIFERA LEAF (UNII: 4WET1AWO9B) INGR ALCOHOL (UNII: 3K9958V90M) INGR SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) INGR DIPROPYLENE GLYCOL (UNII: E107L85C40) INGR GARDEN CRESS SPROUT (UNII: PWQ18YNR62) INGR HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INGR PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR MALTODEXTRIN (UNII: 7CVR7L4A2D) INGR BENZYL SALICYLATE (UNII: WAO5MNK9TU) INGR BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) INGR HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 7.5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 08/22/2022 Part 3 of 4 LONGEVITY CREME
other skin care preparations, leave-on [skin care preparations (creams, lotions, powder, and sprays)]Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR POLYISOBUTYLENE (800000 MW) (UNII: Y132ZOQ9H7) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INGR POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) INGR ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) INGR ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) INGR DIMETHICONE (UNII: 92RU3N3Y1O) INGR PENTYLENE GLYCOL (UNII: 50C1307PZG) INGR DI-PPG-3 MYRISTYL ETHER ADIPATE (UNII: T32481VTXW) INGR STEARYL STEARATE (UNII: 5WX2EGD0DK) INGR PENTAERYTHRITYL DISTEARATE (UNII: 697WOT8HNB) INGR ROYAL JELLY (UNII: L497I37F0C) INGR HONEY (UNII: Y9H1V576FH) INGR HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) INGR MYRISTYL MYRISTATE (UNII: 4042ZC00DY) INGR PALMITOYL GLYCINE (UNII: M6V3RIU5KI) INGR PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR MALTODEXTRIN (UNII: 7CVR7L4A2D) INGR SQUALENE (UNII: 7QWM220FJH) INGR COCONUT OIL (UNII: Q9L0O73W7L) INGR TROLAMINE (UNII: 9O3K93S3TK) INGR CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) INGR EDETATE TRISODIUM (UNII: 420IP921MB) INGR PEG-4 LAURATE (UNII: AYF4VM3N1Z) INGR IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR DIPROPYLENE GLYCOL (UNII: E107L85C40) INGR DMDM HYDANTOIN (UNII: BYR0546TOW) INGR VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) INGR ASCORBYL PALMITATE (UNII: QN83US2B0N) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR BENZYL SALICYLATE (UNII: WAO5MNK9TU) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) INGR HYDROXYCITRONELLOL (UNII: R0B4U2I48W) INGR BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) INGR LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 14 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 01/13/2022 Part 4 of 4 REVITALIZE SOLAR PROTECTION FLUID BROAD SPECTRUM SPF 50
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Item Code (Source) NDC:68828-514 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) KIWI FRUIT OIL (UNII: 66086CWP3Q) ALCOHOL (UNII: 3K9958V90M) ALLANTOIN (UNII: 344S277G0Z) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) ARGININE (UNII: 94ZLA3W45F) DOCOSANOL (UNII: 9G1OE216XY) BENZYL ALCOHOL (UNII: LKG8494WBH) LEVOMENOL (UNII: 24WE03BX2T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SHEA BUTTER (UNII: K49155WL9Y) CHLORPHENESIN (UNII: I670DAL4SZ) COCONUT (UNII: 3RT3536DHY) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIPROPYLENE GLYCOL (UNII: E107L85C40) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) LAUROYL LYSINE (UNII: 113171Q70B) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MALTODEXTRIN (UNII: 7CVR7L4A2D) MORINGA OLEIFERA LEAF (UNII: 4WET1AWO9B) PEG-100 STEARATE (UNII: YD01N1999R) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) POLYGONUM AVICULARE TOP (UNII: ZCD6009IUF) ROYAL JELLY (UNII: L497I37F0C) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) STYRENE/ACRYLAMIDE COPOLYMER (500000 MW) (UNII: 5Z4DPO246A) WATER (UNII: 059QF0KO0R) BENZYL SALICYLATE (UNII: WAO5MNK9TU) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-514-01 14 mL in 1 TUBE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/22/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/22/2022 Labeler - Jafra cosmetics International (041676479) Establishment Name Address ID/FEI Business Operations Distribuidora Comercial Jafra, S.A. de C.V. 951612777 manufacture(68828-511, 68828-512, 68828-513, 68828-514, 68828-515)