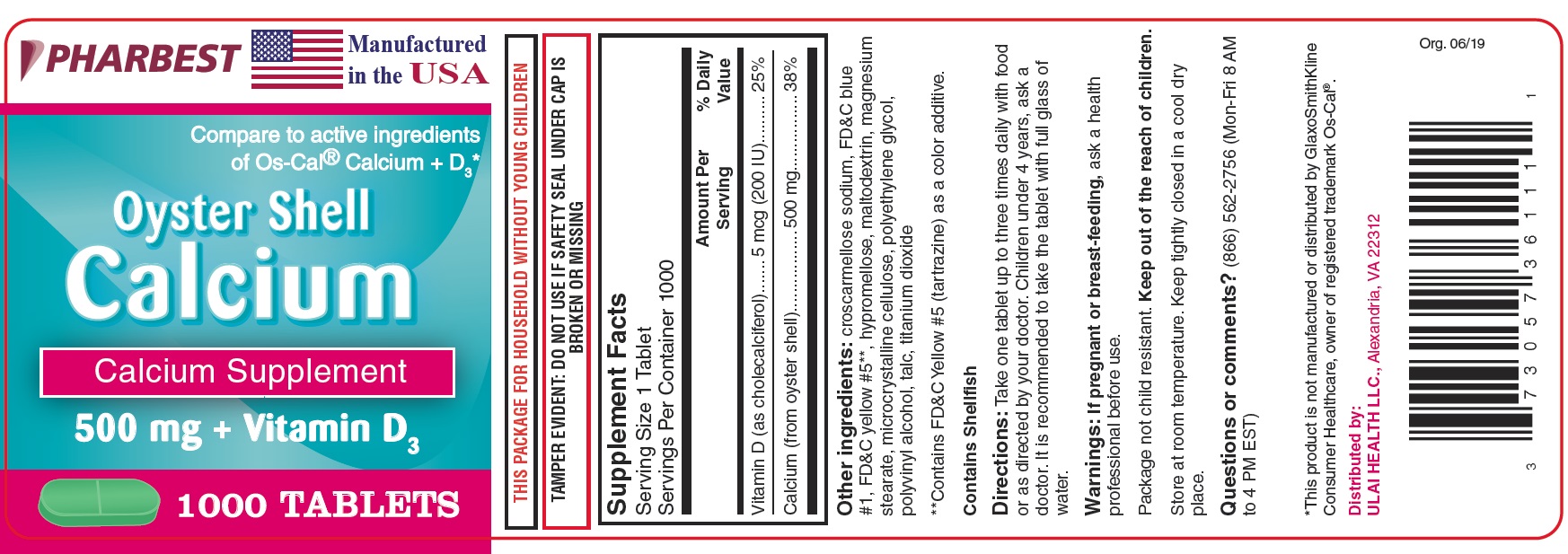
Label: OYSTER SHELL CALCIUM- oyster shell calcium 500mg and vitamin d3 tablet, coated
- NHRIC Code(s): 73057-361-11, 73057-361-07
- Packager: Ulai Health LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated July 22, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Supplement Facts
Serving Size 1 Tablet
Servings Per Container 1000
Amount Per Serving % Daily Value Vitamin D (as cholecalciferol) 5 mcg (200 IU) 25% Calcium (from oyster shell) 500 mg 38% Other ingredients: croscarmellose sodium, FD&C blue #1, FD&C yellow #5**, hypromellose, maltodextrin, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide
**Contains FD&C Yellow #5 (tartrazine) as a color additive.
Contains Shellfish
- Directions:
- Warnings:
- SAFE HANDLING WARNING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OYSTER SHELL CALCIUM
oyster shell calcium 500mg and vitamin d3 tablet, coatedProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:73057-361 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 5 ug CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 500 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MALTODEXTRIN (UNII: 7CVR7L4A2D) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:73057-361-11 1000 in 1 BOTTLE, PLASTIC 2 NHRIC:73057-361-07 60 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 07/22/2019 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 2 shape size (solid drugs) 20 mm Labeler - Ulai Health LLC (081181535) Registrant - Pharbest Pharmaceuticals, Inc. (557054835) Establishment Name Address ID/FEI Business Operations Pharbest Pharmaceuticals, Inc. 557054835 manufacture, analysis, pack, label