Label: LAUNCH MEDICAL- lidocaine hydrochloride cream
- NDC Code(s): 54723-018-01
- Packager: Sambria Pharmaceuticals, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
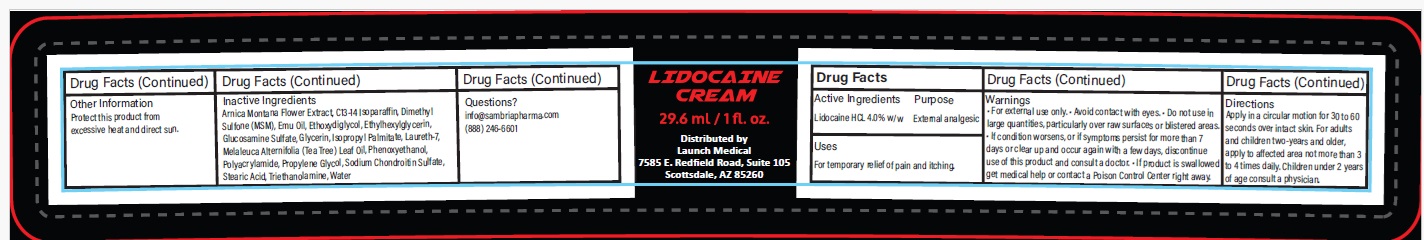
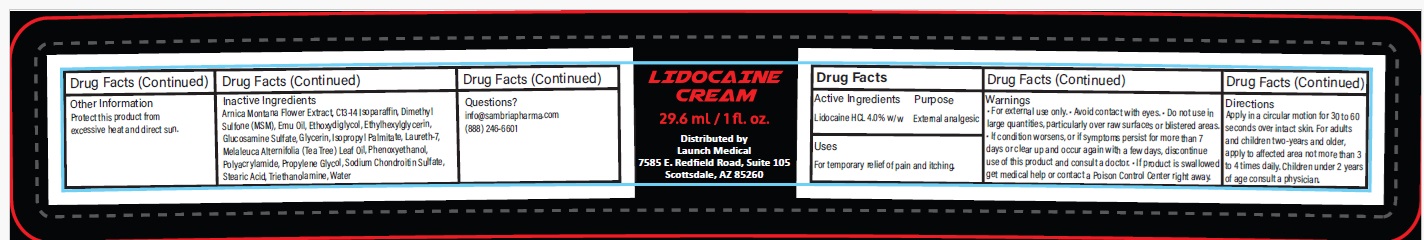
- Drug Facts
- Active ingredient
- Purpose
- Uses
-
Warnings
- For external use only.
- Avoid contact with eyes.
- Do not use in large quantities, particularly over raw surfaces or blistered areas.
- If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again with a few days, discontinue use of this product and consult a doctor.
- If product is swallowed get medical help or contact a Poison Control Center right away.
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
Inactive ingredients
Arnica Montana Flower Extract, C13-14 Isoparaffin, Dimethyl Sulfone (MSM), Emu Oil, Ethoxydiglycol, Ethylhexylglycerin, Glucosamine Sulfate, Glycerin, Isopropyl Palmitate, Laureth-7, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Phenoxyethanol, Polyacrylamide, Propylene Glycol, Sodium Chondroitin Sulfate, Stearic Acid, Triethanolamine, Water
- Other information
- Questions?
- Product label
-
INGREDIENTS AND APPEARANCE
LAUNCH MEDICAL
lidocaine hydrochloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54723-018 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE 4 g in 100 mL Inactive Ingredients Ingredient Name Strength ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) EMU OIL (UNII: 344821WD61) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLUCOSAMINE SULFATE (UNII: 1FW7WLR731) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) LAURETH-7 (UNII: Z95S6G8201) TEA TREE OIL (UNII: VIF565UC2G) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYACRYLAMIDE (10000 MW) (UNII: E2KR9C9V2I) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM CHONDROITIN SULFATE (PORCINE; 5500 MW) (UNII: H5BJH23Z9A) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54723-018-01 30 mL in 1 JAR; Type 0: Not a Combination Product 02/23/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 02/23/2024 Labeler - Sambria Pharmaceuticals, LLC (078676259)