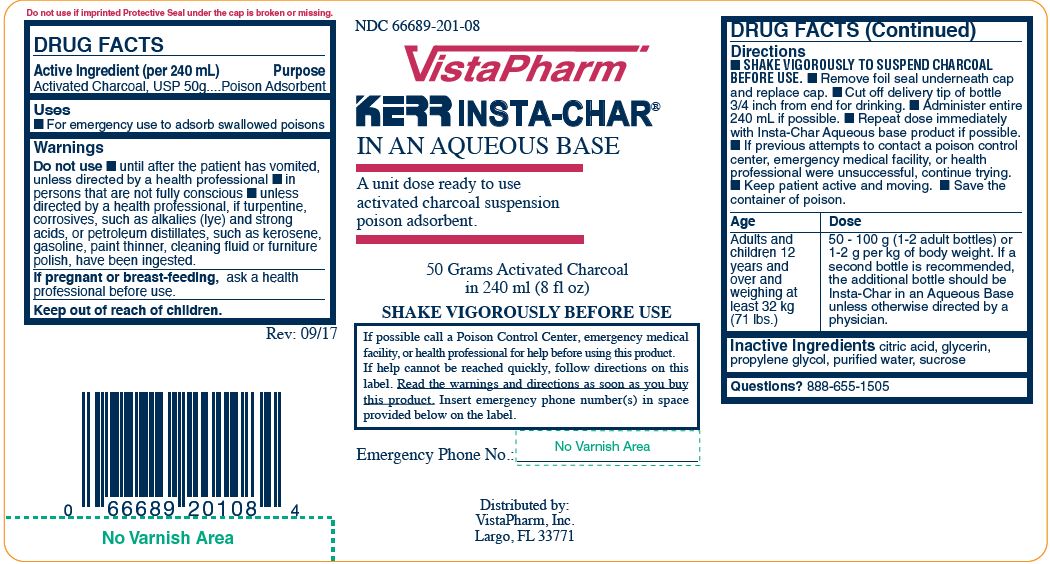
Label: INSTA-CHAR AQUEOUS- poison treatment adsorbent suspension
- NDC Code(s): 66689-201-08
- Packager: VistaPharm, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (per 240 mL)
- Purpose
- Uses
-
Warnings
Do not use
- until after the patient has vomited, unless directed by a health professional
- in persons that are not fully conscious
- unless directed by a health professional, if turpentine, corrosives, such as alkalies (lye) and strong acids, or petroleum distillates, such as kerosene, gasoline, paint thinner, cleaning fluid or furniture polish, have been ingested
-
Directions
- Shake vigorously to suspend charcoal before use.
- Remove foil seal underneath cap and replace cap.
- Cut off delivery tip of bottle 3/4 inch from end for drinking.
- Administer entire 240 mL if possible.
- Repeat dose immediately with Insta-Char Aqueous base product if possible.
- If previous attempts to contact a poison control center. emergency medical facility, of health professional were unsuccessful, continue trying.
- Keep patient active and moving.
- Save the container of poison.
Age Dose Adult and Children 12 years and over and weighing at least 32 kg (71 lbs.) 50-100g (1-2 adult bottles) or 1-2g per kg of body weight. If a second bottle is recommended, the additional bottle should be Insta-Char in an Aqueous Base unless otherwise directed by a physician. - Inactive Ingredients
- Questions?
- Insta-Char Aqueous 240 mL
-
INGREDIENTS AND APPEARANCE
INSTA-CHAR AQUEOUS
poison treatment adsorbent suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66689-201 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 50 g in 240 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66689-201-08 240 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/03/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 10/03/2006 Labeler - VistaPharm, LLC (116743084)