Label: FERROUS SULFATE- ferrous sulfate, dried tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 68210-1520-1, 68210-1520-2, 68210-1520-3 - Packager: SPIRIT PHARMACEUTICALS,LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 9, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients (in each Caplet)
- Purpose
-
Warnings
Do not exceed recommended dosage. The treatment of any anemic condition should be under the advice and supervision of a physician. Since oral iron products interfere with absorption of certain antibiotics, these products should not be taken within tow hours of each other. Occasional gastrointestinal discomfort (such as nausea) may be minimized by taking with meals. Iron-containing products may occasionally cause constipation or diarrhea.
- Direction
- Other information
- Inactive ingredients
-
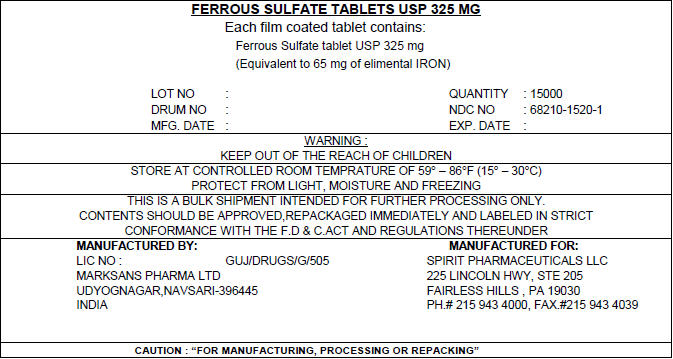
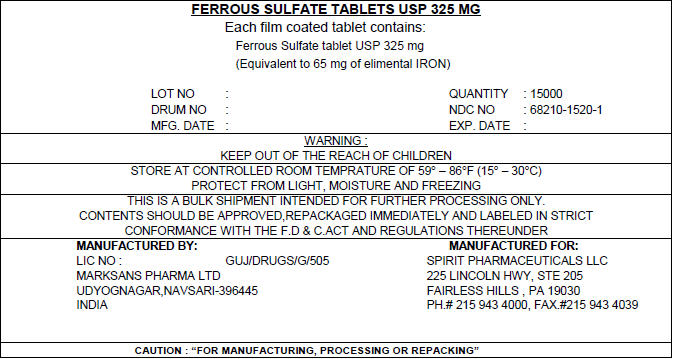
PRINCIPAL DISPLAY PANEL - Shipping Label
FERROUS SULFATE TABLETS USP 325 MG
Each film coated tablet contains:
Ferrous Sulfate tablet USP 325 mg
(Equivalent to 65 mg of elimental IRON)LOT NO : QUANTITY : 15000 DRUM NO : NDC NO : 68210-1520-1 MFG. DATE : EXP. DATE : WARNING :
KEEP OUT OF THE REACH OF CHILDRENSTORE AT CONTROLLED ROOM TEMPRATURE OF 59° – 86°F (15° – 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZINGTHIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED,REPACKAGED IMMEDIATELY AND LABELED IN STRICT
CONFORMANCE WITH THE F.D & C.ACT AND REGULATIONS THEREUNDERMANUFACTURED BY:
LIC NO : GUJ/DRUGS/G/505
MARKSANS PHARMA LTD
UDYOGNAGAR,NAVSARI-396445
INDIAMANUFACTURED FOR:
SPIRIT PHARMACEUTICALS LLC
225 LINCOLN HWY, STE 205
FAIRLESS HILLS , PA 19030
PH.# 215 943 4000, FAX.#215 943 4039CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"
-
INGREDIENTS AND APPEARANCE
FERROUS SULFATE
ferrous sulfate, dried tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68210-1520 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERROUS SULFATE, DRIED (UNII: RIB00980VW) (IRON - UNII:E1UOL152H7) FERROUS SULFATE, DRIED 325 mg Inactive Ingredients Ingredient Name Strength CALCIUM CARBONATE (UNII: H0G9379FGK) GUAR GUM (UNII: E89I1637KE) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CARNAUBA WAX (UNII: R12CBM0EIZ) HYPROMELLOSES (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color GREEN Score no score Shape TRIANGLE Size 14mm Flavor Imprint Code FS Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68210-1520-1 1 in 1 DRUM 1 15000 in 1 BAG 2 NDC:68210-1520-2 1 in 1 DRUM 2 10000 in 1 BAG 3 NDC:68210-1520-3 1 in 1 DRUM 3 1000 in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 12/15/2010 Labeler - SPIRIT PHARMACEUTICALS,LLC (179621011)