Label: RYZNEUTA- efbemalenograstim alfa-vuxw injection
- NDC Code(s): 72893-016-02
- Packager: Acrotech Biopharma Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RYZNEUTA ®safely and effectively. See full prescribing information for RYZNEUTA. RYZNEUTA ®(efbemalenograstim alfa-vuxw ...
-
Table of ContentsTable of Contents
-
1. INDICATIONS AND USAGERYZNEUTA is indicated to decrease the incidence of infection, as manifested by febrile neutropenia, in adult patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs ...
-
2. DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage of RYZNEUTA is a single subcutaneous injection of 20 mg administered once per chemotherapy cycle at least 24 hours after cytotoxic chemotherapy. Do ...
-
3. DOSAGE FORMS AND STRENGTHSInjection: 20 mg/mL clear, colorless, preservative-free solution in a single-dose prefilled syringe.
-
4. CONTRAINDICATIONSRYZNEUTA is contraindicated in patients with a history of serious allergic reactions to granulocyte stimulating factors such as efbemalenograstim alfa-vuxw, pegfilgrastim, or filgrastim products ...
-
5. WARNINGS AND PRECAUTIONS5.1 Splenic Rupture - Splenic rupture, including fatal cases, can occur following the administration of recombinant human granulocyte colony-stimulating factor (rhG-CSF) products, such as ...
-
6. ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling: Splenic Rupture - [see Warnings and Precautions ( 5.1) ...
-
8. USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Although available data with RYZNEUTA use in pregnant women are insufficient to establish whether there is a drug associated risk of major birth defects ...
-
10. OVERDOSAGEOverdosage of RYZNEUTA may result in leukocytosis and bone pain. In the event of overdose, general supportive measures should be instituted, as necessary. Monitor the patient for adverse ...
-
11. DESCRIPTIONEfbemalenograstim alfa-vuxw, a leukocyte growth factor, is a 413 amino acid recombinant fusion protein consisting of human G-CSF, a 16 amino-acid linker, and the Fc portion of human IgG2. In ...
-
12. CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Efbemalenograstim alfa-vuxw is a colony-stimulating factor that acts on hematopoietic cells by binding to specific cell surface receptors, thereby stimulating ...
-
13. NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity or mutagenesis studies have been conducted with efbemalenograstim alfa-vuxw. Efbemalenograstim alfa did not affect ...
-
14. CLINICAL STUDIESThe efficacy of RYZNEUTA to decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anti-cancer drugs was ...
-
16. HOW SUPPLIED/STORAGE AND HANDLINGRYZNEUTA (efbemalenograstim alfa-vuxw) injection is a clear, colorless, preservative-free solution supplied in a prefilled single-dose syringe with a 27-gauge, 1/2-inch needle and an UltraSafe ...
-
17. PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information) Advise patients of the following risks and potential risks with RYZNEUTA: Rupture or enlargement of the spleen ...
-
PATIENT PACKAGE INSERTPatient Information - RYZNEUTA® (rīz-new-ta) (efbemalenograstim alfa-vuxw) injection - Single-Dose Prefilled Syringe - What is RYZNEUTA? RYZNEUTA is a ...
-
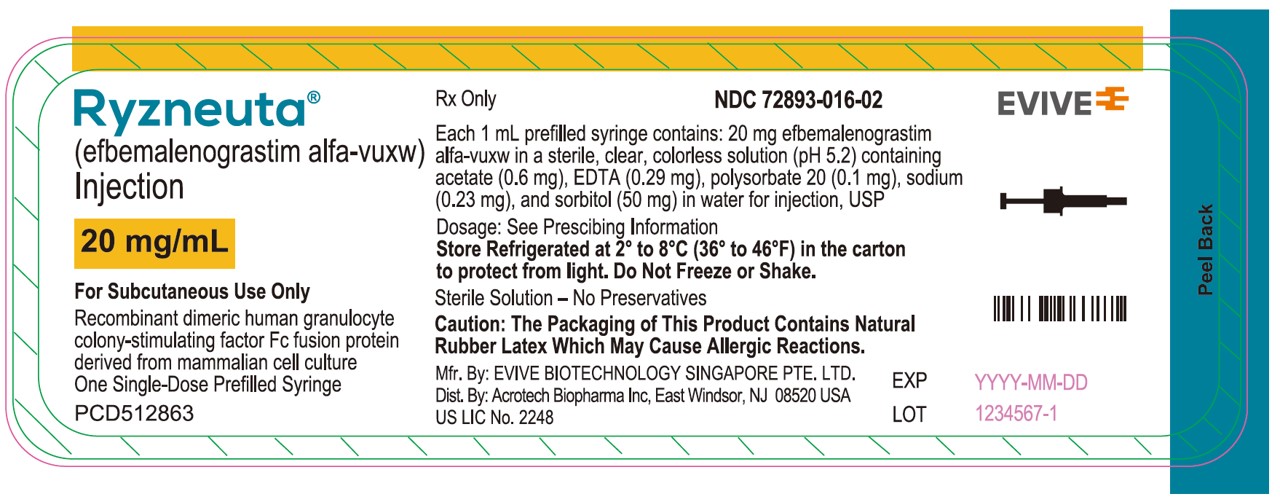
PRINCIPAL DISPLAY PANELSyringe Label - RYZNEUTA - (efbemalenograstim alfa-vuxw) Injection - 20 mg/mL - For Subcutaneous Use Only - Single-dose Prefilled Syringe - Mfr By: EVIVE BIOTECHNOLOGY SINGAPORE PTE. LTD - Dist.By: Acrotech ...
-
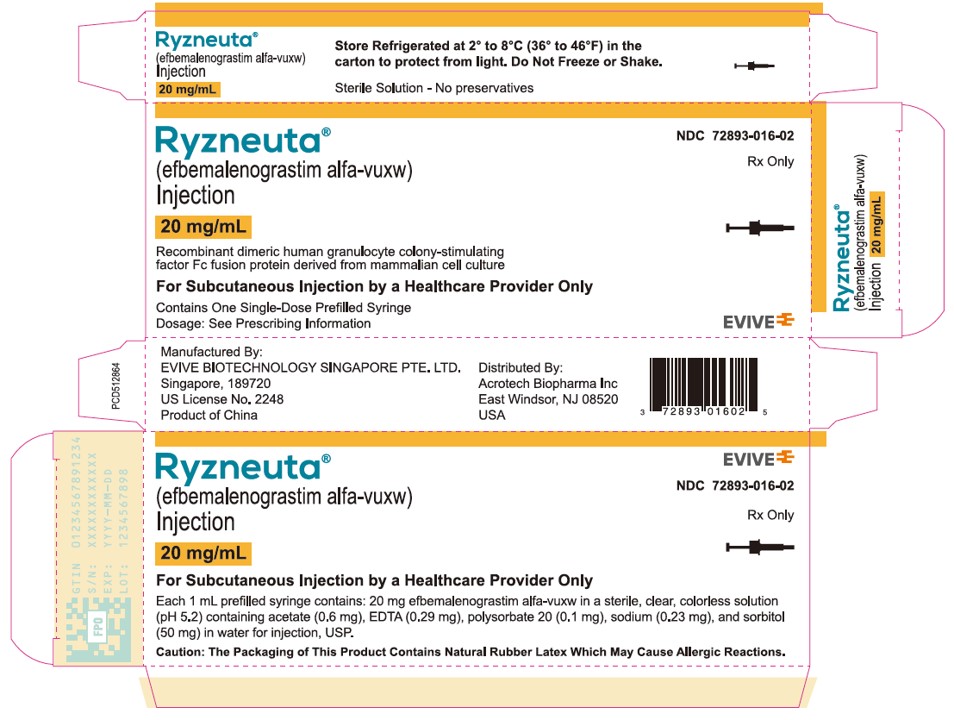
PRINCIPAL DISPLAY PANELCarton Label - RYZNEUTA - (efbemalenograstim alfa-vuxw) Injection - 20 mg/mL - Store refrigerated at 2° to 8°C (36° to 46°F) in the - carton to protect from light. Do Not Freeze or Shake. Sterile ...
-
PRINCIPAL DISPLAY PANELTray Lid Label - RYZNEUTA - (efbemalenograstim alfa-vuxw) Injection - 20 mg/mL - For Subcutaneous Use Only - Recombinant dimeric human granulocyte colony-stimulating factor Fc fusion protein ...
-
INGREDIENTS AND APPEARANCEProduct Information