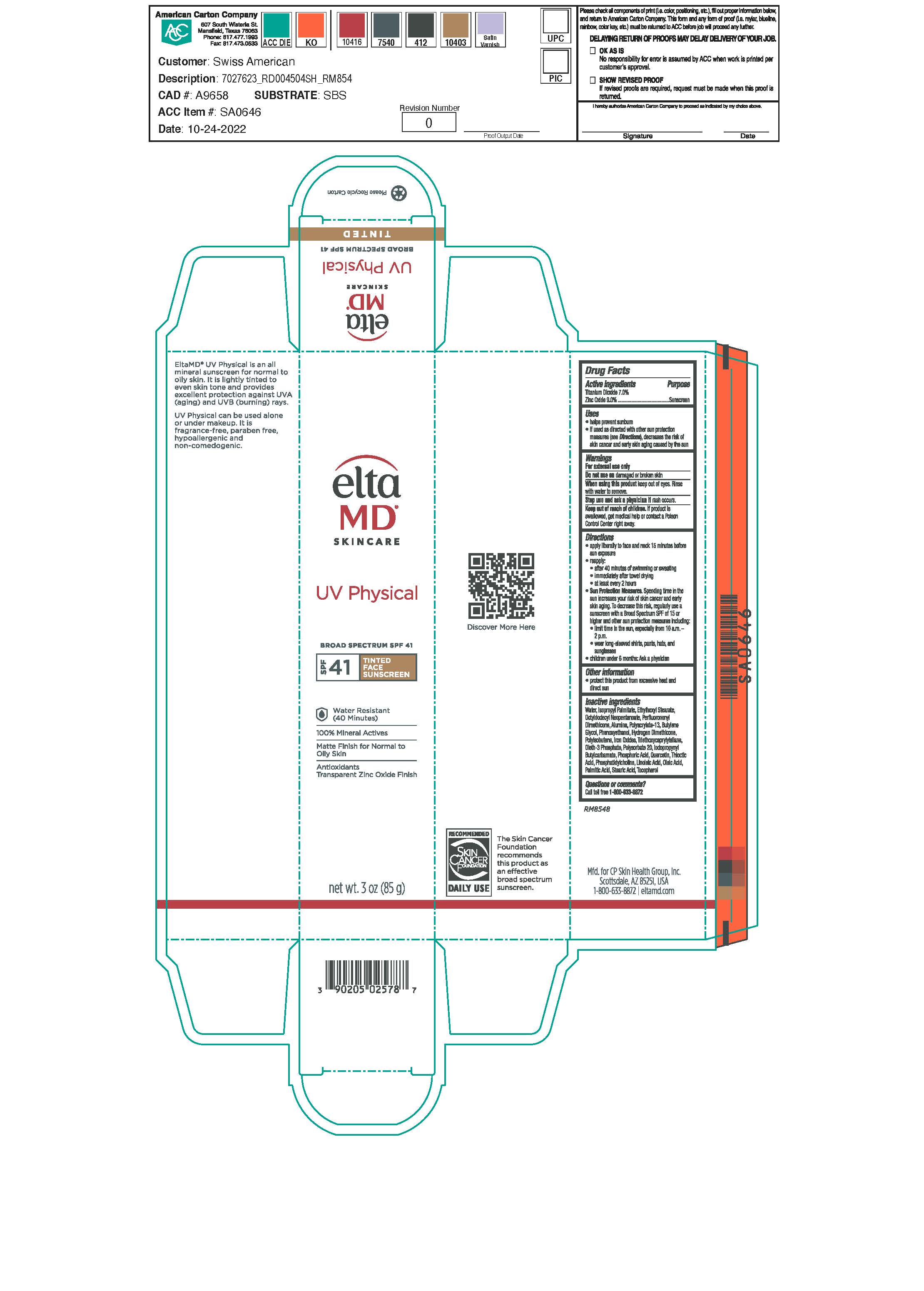
Label: ELTAMD UV PHYSICAL SPF41- zinc oxide and titanium dioxide sunscrreen lotion
- NDC Code(s): 72043-2578-2, 72043-2578-3
- Packager: CP Skin Health Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Warnings
- Active Ingredients
- Uses
- Uses
-
Directions
apply liberally 15 minutes before sun exposure reapply: after 40 minutes of swimming or sweating immediately after towel drying at lest every 2 hours Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF of 15 or higher and other sun protection measures including: limit time int he sun, especially from 10 a.m. - 2 p.m. wear long-sleeve shirts, pants, hats and sunglasses children under 6 months: Ask a physician
-
Inactive Ingredients
Alumina, Butylene Glycol, Citric Acid, Iodopropynyl Butylcarbamate, Iron Oxide, Isopropyl Palmitate, Lecithin, Linoleic Acid, Hydrogen Dimethicone, Octyl Stearate, Octyldodecyl Neopentanoate, Oleth-3 Phosphate, Perfluorononyl Dimethicone, Phenoxyethanol, Polyacrylate 13, Polyisobutene, Polysorbate 20, Purified Water, Quercitin, Sodium Hydroxide, Thioctic Acid, Triethoxycaprylylsilane
- KEEP OUT OF REACH OF CHILDREN
- Labeling
-
INGREDIENTS AND APPEARANCE
ELTAMD UV PHYSICAL SPF41
zinc oxide and titanium dioxide sunscrreen lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72043-2578 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 90 g in 1000 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 70 g in 1000 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) PHENOXYETHANOL (UNII: HIE492ZZ3T) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) OCTYL STEARATE (UNII: 772Y4UFC8B) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) OLETH-3 PHOSPHATE (UNII: 8Q0Z18J1VL) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALUMINUM OXIDE (UNII: LMI26O6933) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) POLYSORBATE 20 (UNII: 7T1F30V5YH) THIOCTIC ACID (UNII: 73Y7P0K73Y) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) QUERCETIN (UNII: 9IKM0I5T1E) LINOLEIC ACID (UNII: 9KJL21T0QJ) Product Characteristics Color brown Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72043-2578-3 85 g in 1 TUBE; Type 0: Not a Combination Product 01/10/2018 2 NDC:72043-2578-2 2 g in 1 PACKET; Type 0: Not a Combination Product 07/06/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/10/2018 Labeler - CP Skin Health Group, Inc. (611921669) Registrant - Swiss-American CDMO, LLC (080170933) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO, LLC 080170933 manufacture(72043-2578)