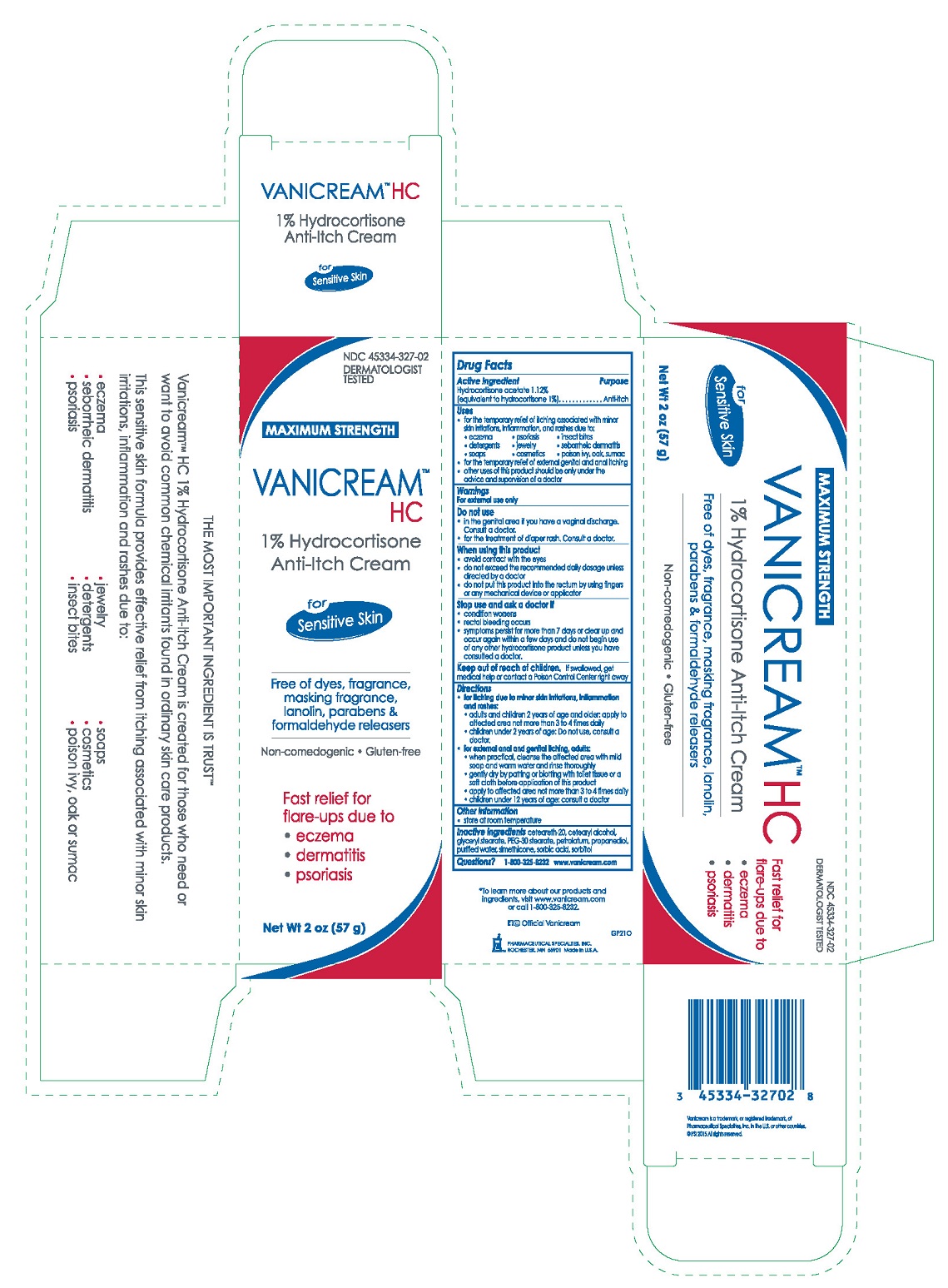
Label: VANICREAM HC ANTI-ITCH- hydrocortisone cream
- NDC Code(s): 45334-327-02, 45334-327-04
- Packager: Pharmaceutical Specialties, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
Uses
- for the temporary relief of itching associated with minor skin irritations, inflammation and rashes due to:
· eczema · psoriasis · insect bites
· detergents · jewelry · seborrheic dermatitis
· soaps · cosmetics · poison ivy, oak, sumac
- for the temporary relief of external genital and anal itching
- other uses of this product should be only under the advice and supervision of a doctor
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- for itching due to minor skin irritations, inflammation and rashes:
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: Do not use, consult a doctor.
- for external anal and genital itching, adults:
- when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly
- gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product
- apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: consult a doctor
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
NDC 45334-327-02
DERMATOLOGIST TESTED
MAXIMUM STRENGTH
VANICREAM™ HC
1% Hydrocortisone Anti-Itch Cream
for Sensitive Skin
Free of dyes, fragrance, masking fragrance, lanolin, parabens & formaldehyde releasers
Non-comedogenic ● Gluten-Free
Fast relief for flare-ups due to
- eczema
- dermatitis
- psoriasis
Net Wt 2 oz (57 g)
-
INGREDIENTS AND APPEARANCE
VANICREAM HC ANTI-ITCH
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:45334-327 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 1.12 g in 100 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) SORBIC ACID (UNII: X045WJ989B) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) PEG-30 STEARATE (UNII: 1U8KB35S20) PROPANEDIOL (UNII: 5965N8W85T) SORBITOL (UNII: 506T60A25R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) WATER (UNII: 059QF0KO0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45334-327-02 1 in 1 CARTON 01/26/2016 1 57 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:45334-327-04 4 g in 1 TUBE; Type 0: Not a Combination Product 02/09/2016 01/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/26/2016 Labeler - Pharmaceutical Specialties, Inc. (076499557) Establishment Name Address ID/FEI Business Operations Pharmaceutical Specialties, Inc. 076499557 manufacture(45334-327) , pack(45334-327) , label(45334-327)