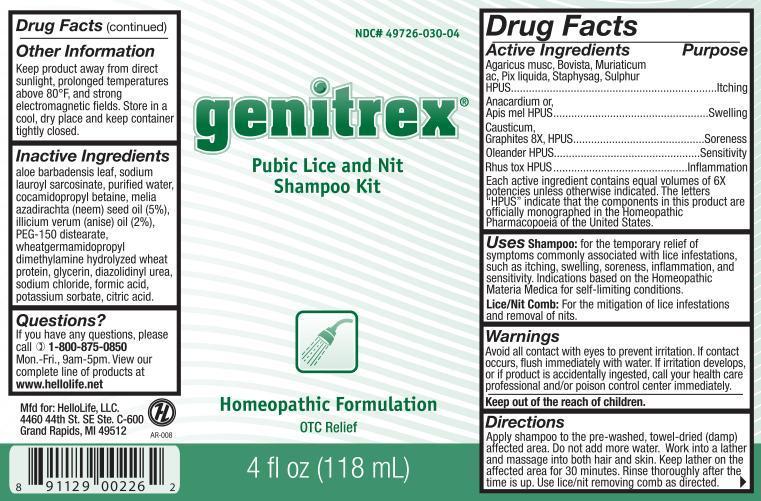
Label: GENITREX- agaricus musc, bovista, muriaticum ac, pix liquida, staphysag, sulphur, anacardium or, apis mel, causticum, graphites, oleander, rhus tox shampoo
-
Contains inactivated NDC Code(s)
NDC Code(s): 49726-030-04 - Packager: Hello Life, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 26, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active Ingredients
Agaricus musc, Bovista, Muriaticum ac, Pix liquida, Staphysag, Sulphur HPUS
Anacardium or, Apis mel HPUS
Causticum, Graphites 8X HPUS
Oleander HPUS
Rhus tox HPUS
Each active ingredient contains equal volumes of 6X potencies unless otherwise indicated. The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
-
Purpose
Agaricus musc, Bovista, Muriaticum ac, Pix liquida, Staphysag, Sulphur HPUS........................Itching
Anacardium or, Apis mel HPUS...........................................................................................Swelling
Causticum, Graphites 8X HPUS.........................................................................................Soreness
Oleander HPUS.............................................................................................................. Sensitivity
Rhus tox HPUS...........................................................................................................Inflammation
-
Uses
Shampoo: For the temporary relief of symptoms commonly associated with lice infestations, such as itching, swelling, soreness, inflammation, and sensitivity. Indications based on the Homeopathic Materia Medica for self-limiting conditions.
Lice/Nit Comb: For the mitigation of lice infestations and removal of nits.
- Warnings
- Directions
- Other Information
-
Inactive ingredients
aloe barbadensis leaf, sodium lauroyl sarcosinate, purified water, cocamidopropyl betaine, melia illicium verum (anise) oil (2%), PEG-150 distearate, wheatgermamidopropyl dimethylamine hydrolyzed wheat protein, glycerin, diazolidinyl urea, sodium chloride, formic acid, potassium sorbate, citric acid - Questions
- DESCRIPTION
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
GENITREX
agaricus musc, bovista, muriaticum ac, pix liquida, staphysag, sulphur, anacardium or, apis mel, causticum, graphites, oleander, rhus tox shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49726-030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMANITA MUSCARIA FRUITING BODY (UNII: DIF093I037) (AMANITA MUSCARIA FRUITING BODY - UNII:DIF093I037) AMANITA MUSCARIA FRUITING BODY 6 [hp_X] in 118 mL LYCOPERDON UTRIFORME FRUITING BODY (UNII: K2A74U428F) (LYCOPERDON UTRIFORME FRUITING BODY - UNII:K2A74U428F) LYCOPERDON UTRIFORME FRUITING BODY 6 [hp_X] in 118 mL HYDROCHLORIC ACID (UNII: QTT17582CB) (HYDROCHLORIC ACID - UNII:QTT17582CB) HYDROCHLORIC ACID 6 [hp_X] in 118 mL PINE TAR (UNII: YFH4WC535J) (PINE TAR - UNII:YFH4WC535J) PINE TAR 6 [hp_X] in 118 mL DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 6 [hp_X] in 118 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 6 [hp_X] in 118 mL SEMECARPUS ANACARDIUM JUICE (UNII: Y0F0BU8RDU) (SEMECARPUS ANACARDIUM JUICE - UNII:Y0F0BU8RDU) SEMECARPUS ANACARDIUM JUICE 6 [hp_X] in 118 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 6 [hp_X] in 118 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 6 [hp_X] in 118 mL GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 8 [hp_X] in 118 mL NERIUM OLEANDER LEAF (UNII: 7KV510R6H6) (NERIUM OLEANDER LEAF - UNII:7KV510R6H6) NERIUM OLEANDER LEAF 6 [hp_X] in 118 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 6 [hp_X] in 118 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) AZADIRACHTA INDICA SEED OIL (UNII: 4DKJ9B3K2T) WATER (UNII: 059QF0KO0R) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) GLYCERIN (UNII: PDC6A3C0OX) STAR ANISE OIL (UNII: 6RXP35EIRE) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) LAVENDER OIL (UNII: ZBP1YXW0H8) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) FORMIC ACID (UNII: 0YIW783RG1) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49726-030-04 1 in 1 CARTON 02/04/2013 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/04/2013 Labeler - Hello Life, Inc. (065619378) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 manufacture(49726-030)