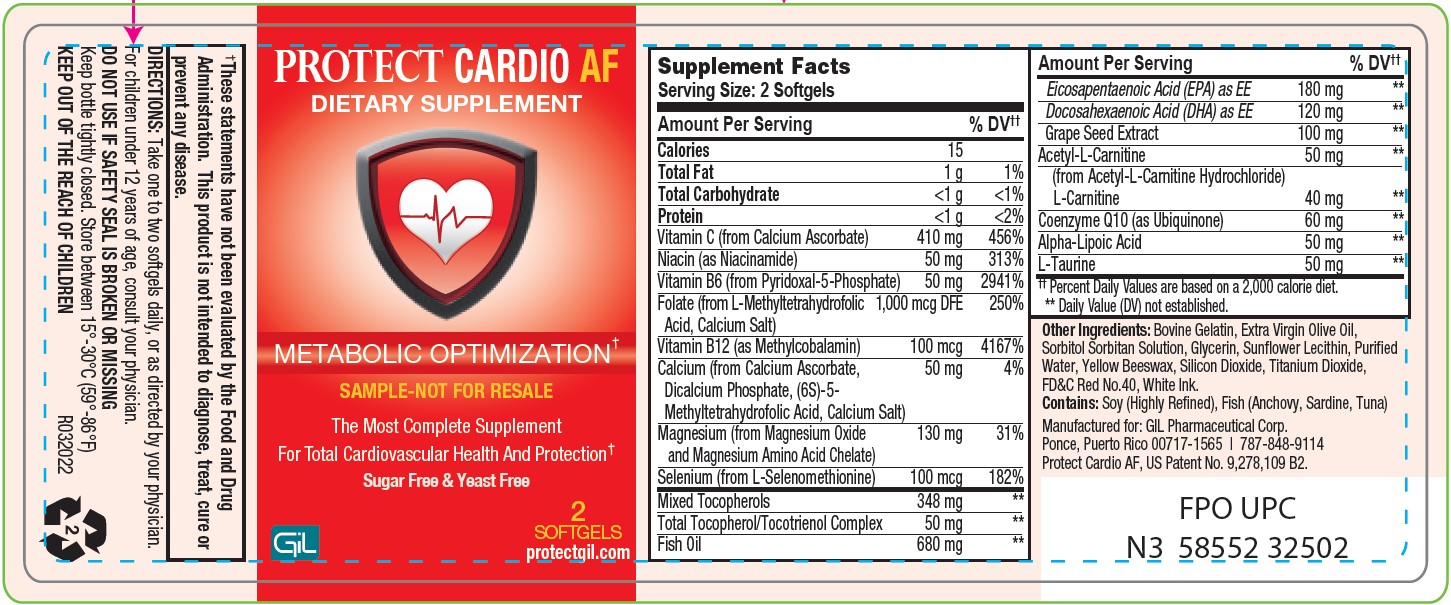
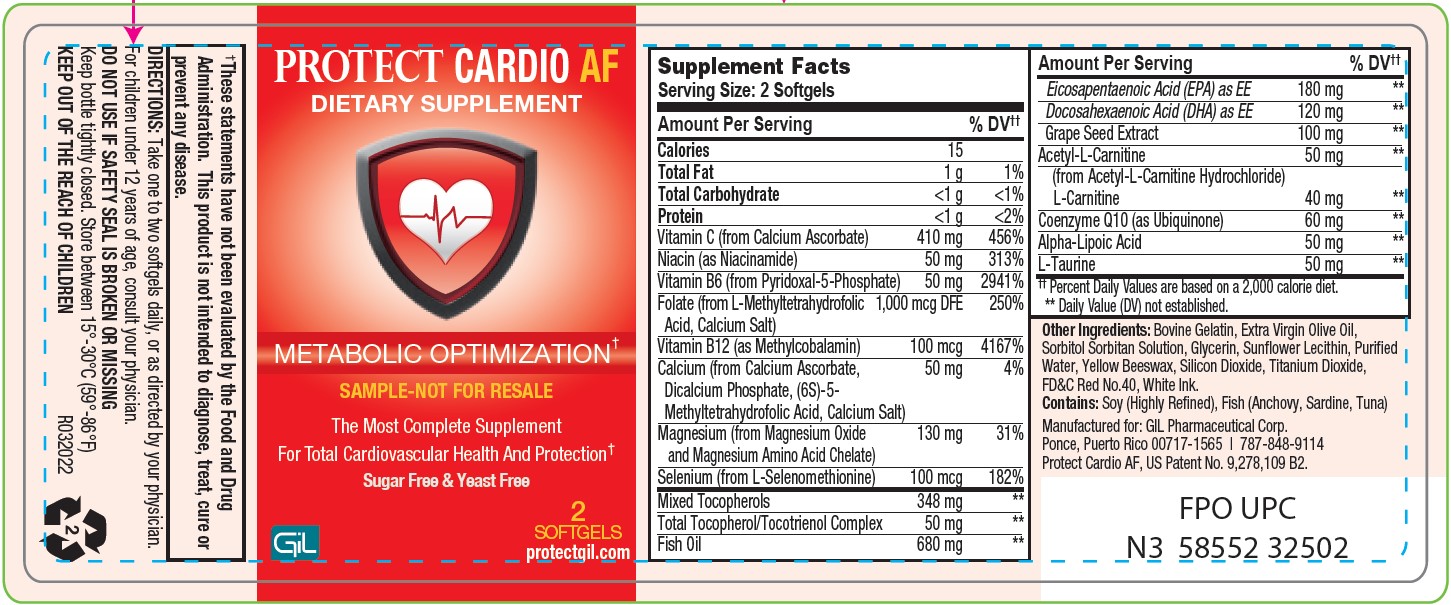
Label: PROTECT CARDIO AF- vitamin c, niacin, vitamin b6, folate, vitamin b12, magnesium, selenium, tocopherols, fish oil, grape seed extract, acetyl-l-carnitine, l-carnitine, trimethylglycine, coenzyme q10, alpha-lipoic acid, l-taurine capsule
- NHRIC Code(s): 58552-325-02, 58552-325-60
- Packager: GIL Pharmaceutical Corp.
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated November 7, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- STATEMENT OF IDENTITY
-
SUPPLEMENT FACTS
Serving Size 2 Softgels
Servings Per Container: 30
*Percent Dally Values are based on a 2000 Calorie Diet.
**Daily Value not established.
Amount Per Serving %DV Calories 15 Total Fat 1g 1% Total Carbohydrate <1 g <1% Protein <1 g 2% Vitamin C (from Calcium ascorbate) 500 mg 556% Niacin (as Niacinamide) 50 mg 313% Vitamin B6 (from Pyridoxal-5-Phosphate) 50 mg 2941% Folate (from L-Methyltetrahydrofolic Acid,
Calcium Salt)1000 mcg DFE 250% Vitamin B12 (as Methylcobalamin) 100 mcg 4167% Magnesium (from Magnesium Oxide and Magnesium Amino Acid Chelate) 200 mg 48% Selenium (from L-Selenomethionine) 100 mcg 182% Mixed Tocopherols 348 mg ** Total Tocopherol/Tocotrienol Complex 50 mg ** Fish Oil 680 mg ** Eicosapentaenoic Acid (EPA) as EE 180 mg ** Docosahexaenoic Acid (DHA) as EE 120 mg ** Grape Seed Extract 100 mg ** Acetyl-L-Carnilite (from Acetyl-L-Carnilite Hydrochloride) 125 mg ** L- Carnilite 100 mg ** Trimethylglycine (from Betaine Hydrochloride) 100 mg ** Coenzyme Q10 (as Ubiquinone) 60 mg ** Alpha-Lipoic Acid 50 mg ** L-Taurine 50 mg ** - Other Ingredients
- WARNINGS
- DOSAGE & ADMINISTRATION
- DO NOT USE IF SAFETY SEAL IS BROKEN OR MISSING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PROTECT CARDIO AF
vitamin c, niacin, vitamin b6, folate, vitamin b12, magnesium, selenium, tocopherols, fish oil, grape seed extract, acetyl-l-carnitine, l-carnitine, trimethylglycine, coenzyme q10, alpha-lipoic acid, l-taurine capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:58552-325 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 500 mg NIACIN (UNII: 2679MF687A) (NIACIN - UNII:2679MF687A) NIACIN 50 mg PYRIDOXINE (UNII: KV2JZ1BI6Z) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 50 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg METHYLCOBALAMIN (UNII: BR1SN1JS2W) (METHYLCOBALAMIN - UNII:BR1SN1JS2W) METHYLCOBALAMIN 0.1 mg MAGNESIUM (UNII: I38ZP9992A) (MAGNESIUM - UNII:I38ZP9992A) MAGNESIUM 200 mg SELENIUM (UNII: H6241UJ22B) (SELENIUM - UNII:H6241UJ22B) SELENIUM 0.1 mg TOCOPHEROL (UNII: R0ZB2556P8) (TOCOPHEROL - UNII:R0ZB2556P8) TOCOPHEROL 348 mg TOCOTRIENOL (UNII: 0867I0N41V) (TOCOTRIENOL - UNII:0867I0N41V) TOCOTRIENOL 50 mg FISH OIL (UNII: XGF7L72M0F) (FISH OIL - UNII:XGF7L72M0F) FISH OIL 680 mg ICOSAPENT (UNII: AAN7QOV9EA) (ICOSAPENT - UNII:AAN7QOV9EA) ICOSAPENT 180 mg DOCONEXENT (UNII: ZAD9OKH9JC) (DOCONEXENT - UNII:ZAD9OKH9JC) DOCONEXENT 120 mg VITIS VINIFERA SEED (UNII: C34U15ICXA) (VITIS VINIFERA SEED - UNII:C34U15ICXA) VITIS VINIFERA SEED 100 mg ACETYLCARNITINE (UNII: 6DH1W9VH8Q) (LEVOCARNITINE - UNII:0G389FZZ9M) ACETYLCARNITINE 125 mg LEVOCARNITINE (UNII: 0G389FZZ9M) (LEVOCARNITINE - UNII:0G389FZZ9M) LEVOCARNITINE 100 mg BETAINE (UNII: 3SCV180C9W) (BETAINE - UNII:3SCV180C9W) BETAINE 100 mg UBIDECARENONE (UNII: EJ27X76M46) (UBIDECARENONE - UNII:EJ27X76M46) UBIDECARENONE 60 mg ALPHA LIPOIC ACID (UNII: 73Y7P0K73Y) (.ALPHA.-LIPOIC ACID - UNII:73Y7P0K73Y) ALPHA LIPOIC ACID 50 mg TAURINE (UNII: 1EQV5MLY3D) (TAURINE - UNII:1EQV5MLY3D) TAURINE 50 mg Inactive Ingredients Ingredient Name Strength ALPHA-LACTALBUMIN (BOVINE) (UNII: Z62V84ZZ4G) GLYCERIN (UNII: PDC6A3C0OX) OLIVE OIL (UNII: 6UYK2W1W1E) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) YELLOW WAX (UNII: 2ZA36H0S2V) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C RED NO. 40 (UNII: WZB9127XOA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:58552-325-02 2 in 1 BOTTLE 2 NHRIC:58552-325-60 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 11/07/2022 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color size (solid drugs) 3 mm scoring 1 shape Labeler - GIL Pharmaceutical Corp. (176826592)