Label: BSS- balanced salt solution solution
- NDC Code(s): 0065-1795-04
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
BSS™ Sterile Irrigating Solution is a sterile balanced salt solution, each mL containing sodium chloride (NaCl) 0.64%, potassium chloride (KCl) 0.075%, calcium chloride dihydrate (CaCl2•2H2O) 0.048%, magnesium chloride hexahydrate (MgCl2•6H2O) 0.03%, sodium acetate trihydrate (C2H3NaO2•3H2O) 0.39%, sodium citrate dihydrate (C6H5Na3O7•2H2O) 0.17%, sodium hydroxide and/or hydrochloric acid (to adjust pH), and water for injection. The pH is approximately 7.5. The osmolality is approximately 300 mOsm/Kg.
- CLINICAL PHARMACOLOGY:
- INDICATIONS AND USAGE:
-
WARNINGS:
- NOT FOR INJECTION OR INTRAVENOUS INFUSION.
- Do not use unless product is clear, seal is intact, and container is undamaged.
- Do not use if product is discolored or contains a precipitate.
- SINGLE patient use only. The contents of this bag should not be used in more than one patient.
- This solution contains no preservative, unused contents should be discarded.
-
PRECAUTIONS:
Open under aseptic conditions only.
Studies suggest that intraocular irrigating solutions which are iso-osmotic with normal aqueous fluids should be used with caution in diabetic patients undergoing vitrectomy since intraoperative lens changes have been observed.
There have been reports of corneal clouding and edema following ocular surgery in which BSS Sterile Irrigating Solution was used as an irrigating solution.
- ADVERSE REACTIONS:
-
DOSAGE AND ADMINISTRATION:
This irrigating solution should be used according to standard format for each surgical procedure. Follow directions of the particular administration set to be used. Remove the outer overwrap. Clean and disinfect the rubber stopper by using a sterile alcohol wipe. Insert the spike aseptically into the bag through the target area of the rubber stopper. Allow the fluid to flow and remove air from the tubing before irrigation begins.
-
HOW SUPPLIED:
BSS™ Sterile Irrigating Solution is supplied in a clear polypropylene bag using a grey butyl stopper and aluminum seal packaged in a clear, multilayered plastic outer overwrap.
500 mL in a bag: NDC 0065-1795-04.
STORAGE: Store at 36° - 77°F (2° - 25°C).
Alcon
Distributed by:
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
© 2021 Alcon, Inc.
-
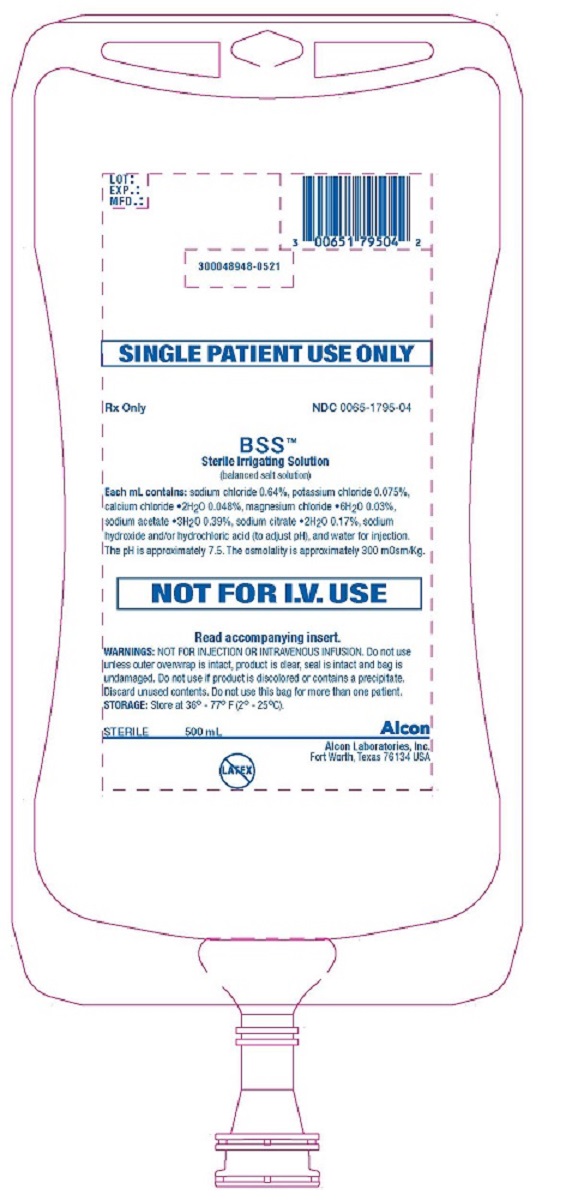
PRINCIPAL DISPLAY PANEL
SINGLE PATIENT USE ONLY
Rx Only
NDC 0065-1795-04
BSS™
Sterile Irrigating Solution
(balanced salt solution)
Each mL contains: sodium chloride 0.64%, potassium chloride 0.075%, calcium chloride •2H2O 0.048%, magnesium chloride •6H2O 0.03%, sodium acetate •3H2O 0.39%, sodium citrate •2H2O 0.17%, sodium hydroxide and/or hydrochloric acid (to adjust pH), and water for injection. The pH is approximately 7.5. The osmolality is approximately 300 mOsm/Kg.
NOT FOR I.V. USE
Read accompanying insert.
WARNINGS: NOT FOR INJECTION OR INTRAVENOUS INFUSION. Do not use unless outer overwrap is intact, product is clear, seal is intact and bag is undamaged. Do not use if product is discolored or contains a precipitate. Discard unused contents. Do not use this bag for more than one patient.
STORAGE: Store at 36° - 77° F (2° - 25°C).
STERILE 500 mL
Alcon
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
LOT:
EXP.:
MFD.:
300048948-0521
Storage: Store at 36° - 77°F (2° - 25°C).
Do not use unless outer overwrap is intact.
9012629-1115

-
INGREDIENTS AND APPEARANCE
BSS
balanced salt solution solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0065-1795 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 6.4 mg in 1 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 0.75 mg in 1 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CHLORIDE 0.48 mg in 1 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CHLORIDE 0.3 mg in 1 mL SODIUM ACETATE (UNII: 4550K0SC9B) (Sodium Cation - UNII:LYR4M0NH37) SODIUM ACETATE 3.9 mg in 1 mL SODIUM CITRATE (UNII: 1Q73Q2JULR) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CITRATE 1.7 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-1795-04 500 mL in 1 BAG; Type 0: Not a Combination Product 03/28/1969 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020742 03/28/1969 Labeler - Alcon Laboratories, Inc. (008018525) Registrant - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(0065-1795)