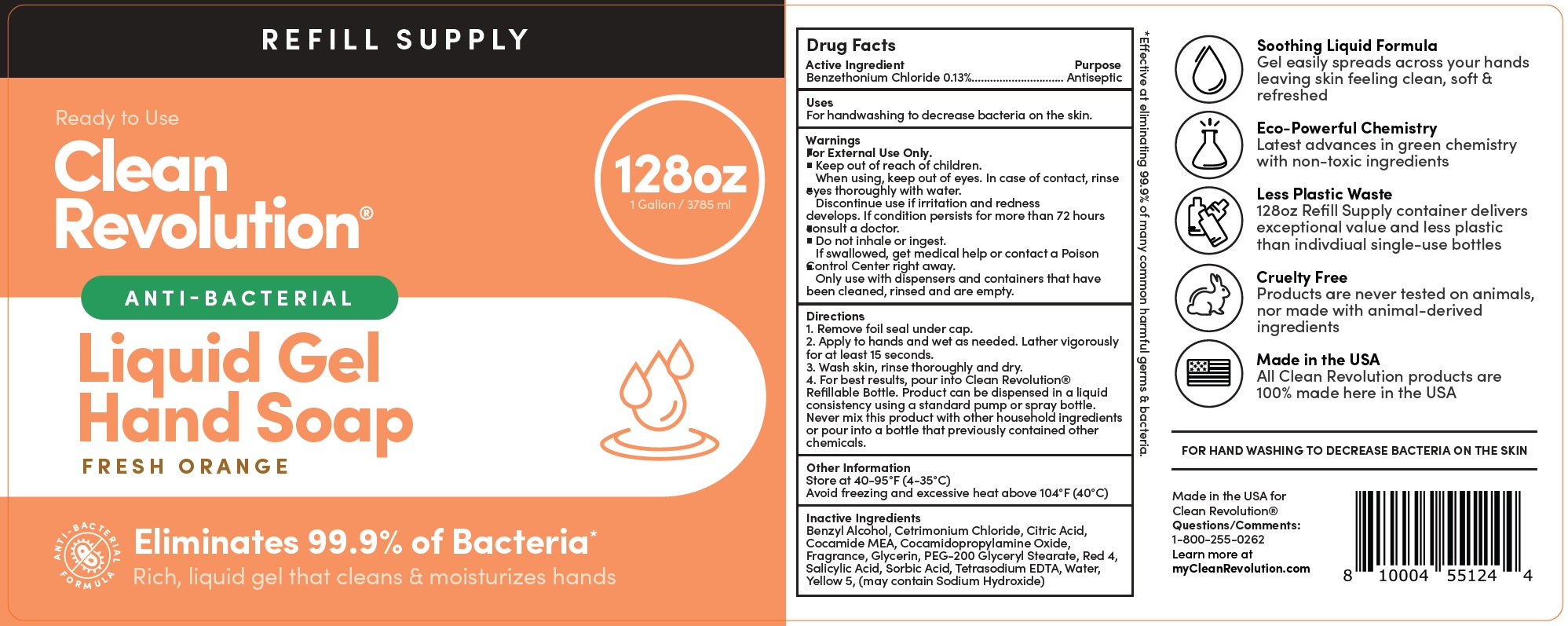
Label: CLEAN REVOLUTION ANTI-BACTERIAL HAND REFILL SUPPLY CONTAINER - ANTI-BACTERIAL - ELIMINATES 99.9% GERMS - READY TO USE - FRESH ORANGE- benzethonium chloride liquid
- NDC Code(s): 74810-728-00
- Packager: Replenish Bottling LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses
-
Warnings
For External Use Only.
When using,
- keep out of eyes. In case of contact, rinse eyes thoroughly with water.
- Discontinue use if irritation and redness develops. If condition persists for more than 72 hours consult a doctor.
- Do not inhale or ingest.
- If swallowed, get medical help or contact a Poison Control Center right away.
- Only use with dispensers and containers that have been cleaned, rinsed and are empty.
-
Directions
1. Remove foil seal under cap.
2. Apply to hands and wet as needed. Lather vigorously for at least 15 seconds.
3. Wash skin, rinse thoroughly and dry.
4. For best results, pour into Clean Revolution® Refillable Bottle. Product can be dispensed in a liquid consistency using a standard pump or spray bottle. Never mix this product with other household ingredients or pour into a bottle that previously contained other chemicals. - Other Information
- Inactive Ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
CLEAN REVOLUTION ANTI-BACTERIAL HAND REFILL SUPPLY CONTAINER - ANTI-BACTERIAL - ELIMINATES 99.9% GERMS - READY TO USE - FRESH ORANGE
benzethonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74810-728 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) COCO MONOETHANOLAMIDE (UNII: C80684146D) COCAMIDOPROPYLAMINE OXIDE (UNII: M4SL82J7HK) GLYCERIN (UNII: PDC6A3C0OX) PEG-200 GLYCERYL STEARATE (UNII: Z8ROG2A0LZ) FD&C RED NO. 4 (UNII: X3W0AM1JLX) SALICYLIC ACID (UNII: O414PZ4LPZ) SORBIC ACID (UNII: X045WJ989B) EDETATE SODIUM (UNII: MP1J8420LU) WATER (UNII: 059QF0KO0R) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74810-728-00 3785 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 06/01/2023 Labeler - Replenish Bottling LLC (044586187)