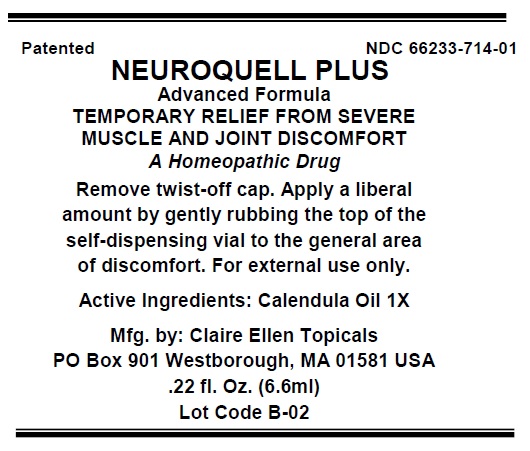
Label: NEUROQUELL-PLUS oil
-
Contains inactivated NDC Code(s)
NDC Code(s): 66233-714-01 - Packager: Atlantic Management Resources Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 11, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- WARNINGS
- INACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
-
DOSAGE & ADMINISTRATION SECTION
When you are ready to apply Neuroquell, remove the cap, turn the bottle upside down, and gently roll the applicator onto the area of discomfort in a continuous, back-and-forth motion to cover approximately one-half of the area of your pain. If using either the sample swab or towelette, apply in a similar way. Your skin will remain moist for a few minutes. Close cap tightly after use, and wash hands thoroughly. Feel free to reapply 4-to-6 hours later and up to 3 additional times per day. To eliminate the peppermint scent, you can wash the treated area with soap and water 15 minutes after the application. This will not affect the efficacy of the product. If there is highlights text then the SPL document title should include the following text string without quotation marks: These highlights do not include all the information needed to use see full prescribing information for and Initial U.S. Approval
- INDICATIONS & USAGE SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NEUROQUELL-PLUS
neuroquell-plus oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66233-714 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) 0.001 g in 1 g ALMOND OIL (UNII: 66YXD4DKO9) 0.005 g in 1 g MENTHA ARVENSIS LEAF OIL (UNII: 1AEY1M553N) 0.82 g in 1 g ORANGE OIL (UNII: AKN3KSD11B) 0.007 g in 1 g CAMPHOR OIL, WHITE (UNII: 26P3H26Z9X) 0.05 g in 1 g CORIANDER OIL (UNII: 7626GC95E5) 0.002 g in 1 g PENNYROYAL OIL (UNII: AK85U7Y3MV) 0.04 g in 1 g ROSEMARY OIL (UNII: 8LGU7VM393) 0.06 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66233-714-01 1 g in 1 BOTTLE, WITH APPLICATOR; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 07/04/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/05/2004 Labeler - Atlantic Management Resources Inc (959409855) Registrant - Atlantic Management Resources Inc (959409855) Establishment Name Address ID/FEI Business Operations Atlantic Management Resources Inc 959409855 manufacture(66233-714)