Label: DENTEK EUGENOL- eugenol liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 60630-566-01, 60630-566-02 - Packager: DenTek Oral Care, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 28, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
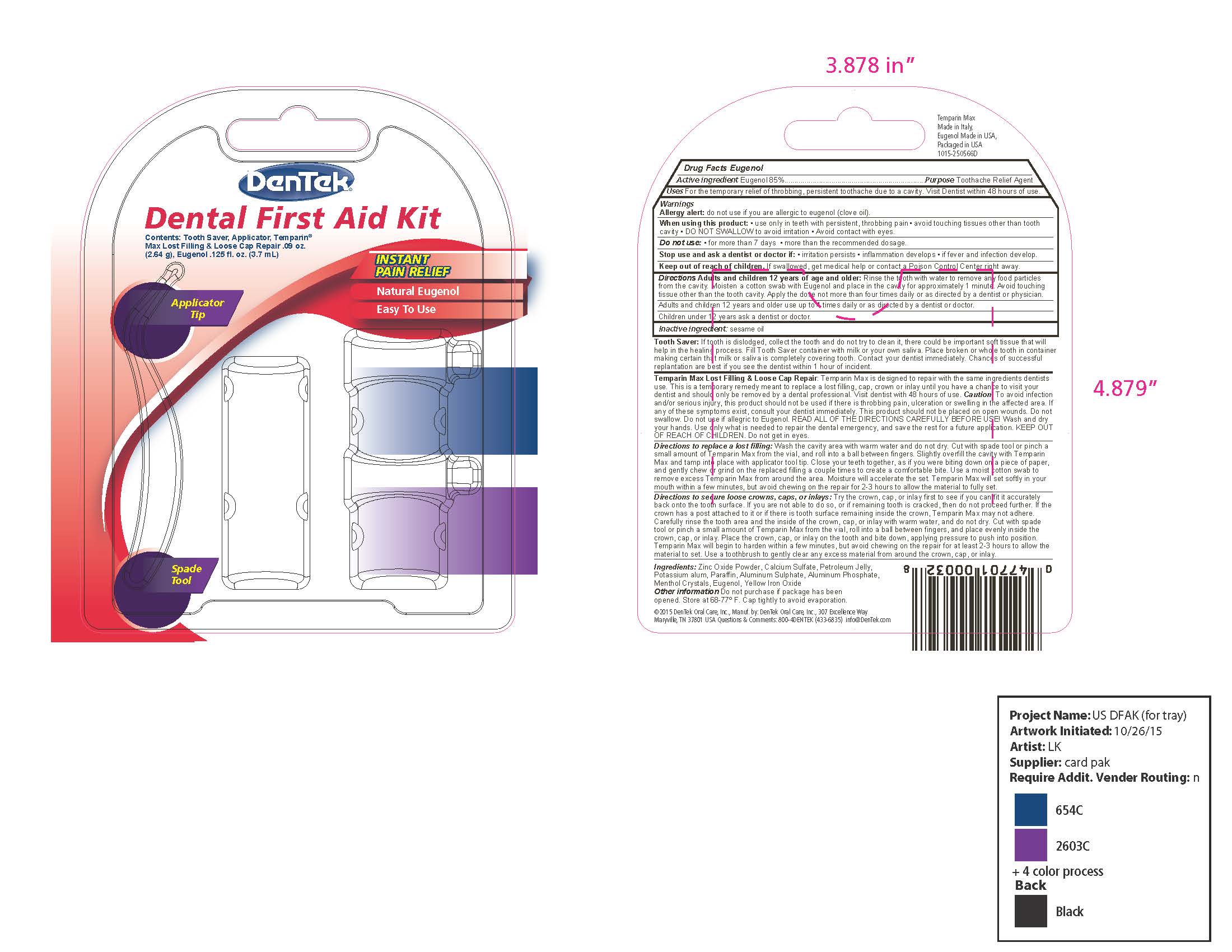
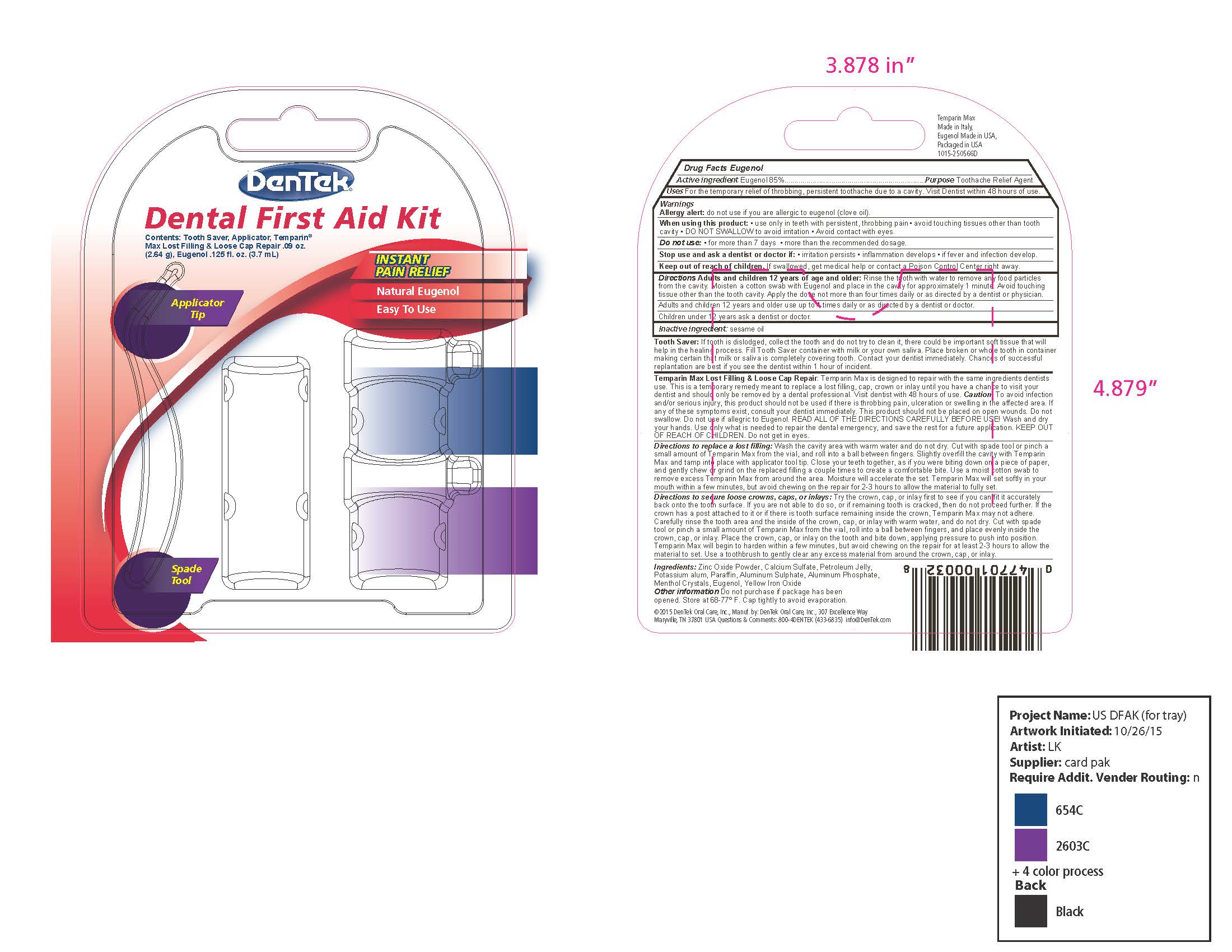
Directions Adults and children 12 year of age and older: Rinse the tooth with water to remove any food particles from the cavity. Moisten a cotton swab with Eugenol and place in the cavity for approximately 1 minute. Avoid touching tissue other than the tooth cavity. Apply the dose not more than four times daily or as directed by dentist or physician.
Adults and children 12 years and older use up to 4 times daily or as directed by a dentist or doctor.
Children under 12 years ask a dentist or doctor.
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- Outer Retail Package Label - DenTek Dental First Aid Kit
-
INGREDIENTS AND APPEARANCE
DENTEK EUGENOL
eugenol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60630-566 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUGENOL (UNII: 3T8H1794QW) (EUGENOL - UNII:3T8H1794QW) EUGENOL 850 mg in 1 mL Inactive Ingredients Ingredient Name Strength SESAME OIL (UNII: QX10HYY4QV) 150 mg in 1 mL Product Characteristics Color yellow (Clear to pale yellow liquid, with odor of cloves) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60630-566-02 1 in 1 KIT 07/01/2015 1 NDC:60630-566-01 3.7 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 07/01/2015 Labeler - DenTek Oral Care, Inc. (153818646) Registrant - DenTek Oral Care, Inc. (153818646) Establishment Name Address ID/FEI Business Operations Polysciences, Inc. 014249635 manufacture(60630-566) Establishment Name Address ID/FEI Business Operations DenTek Oral Care, Inc. 153818646 label(60630-566)