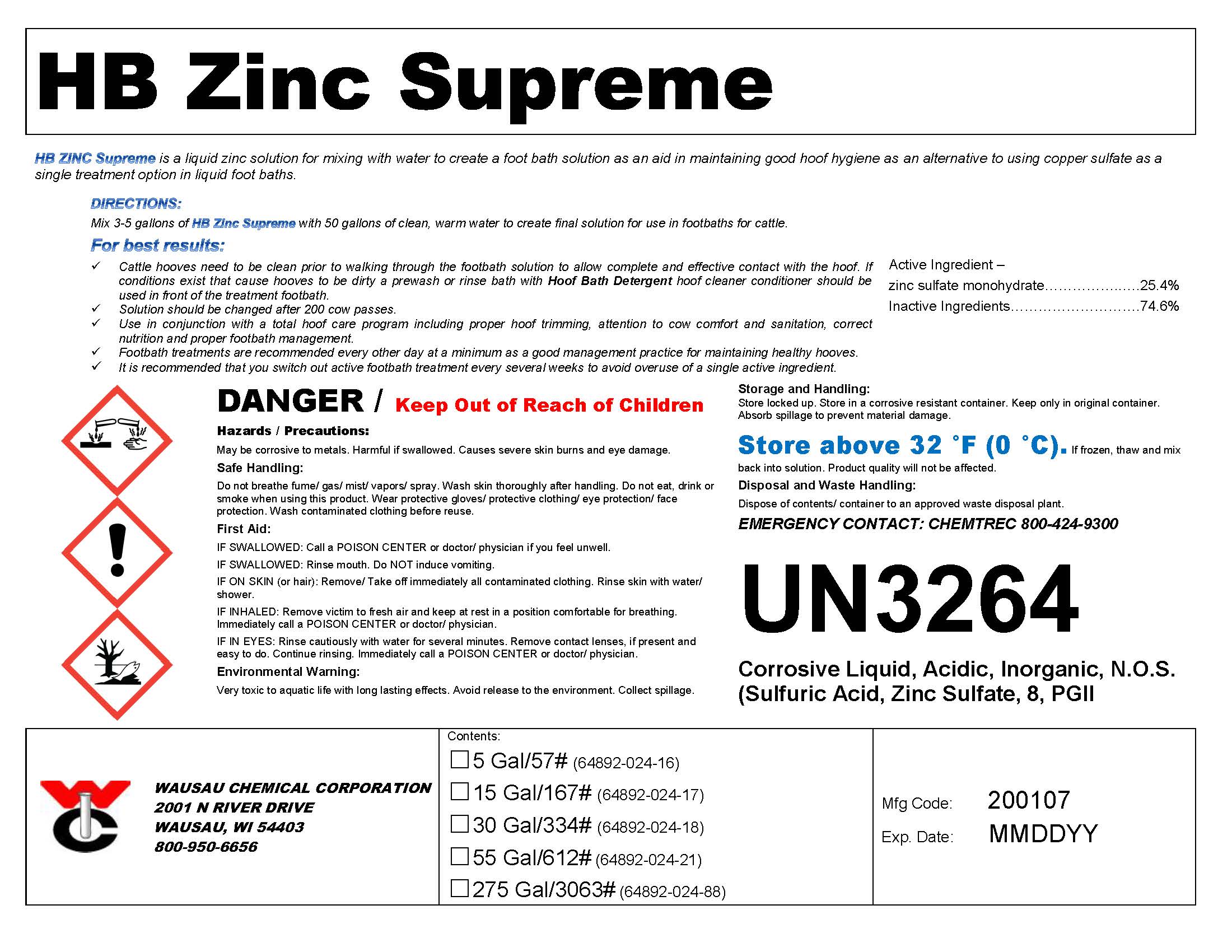
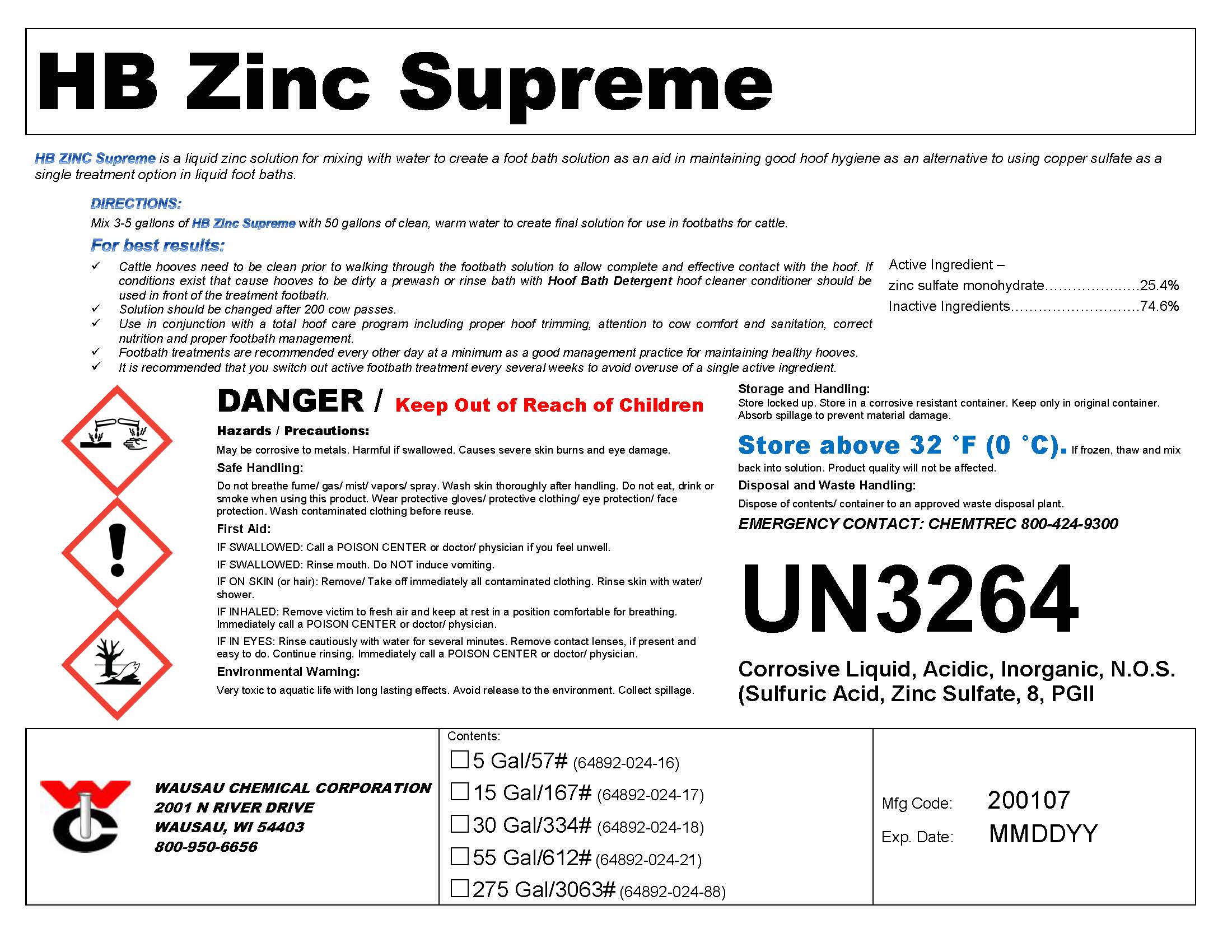
Label: HB ZINC SUPREME- zinc footbath solution
-
NDC Code(s):
64892-024-16,
64892-024-17,
64892-024-18,
64892-024-21, view more64892-024-88
- Packager: Wausau Chemical
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 7, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DISPOSAL AND WASTE HANDLING
- ENVIRONMENTAL WARNING
- GENERAL PRECAUTIONS
-
First Aid:
IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF ON SKIN (or hair): Remove/ Take off immediately all contaminated clothing. Rinse skin with water/ shower.
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. Immediately call a POISON CENTER or doctor/ physician.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER or doctor/ physician. - SAFE HANDLING WARNING
- STORAGE AND HANDLING
-
VETERINARY INDICATIONS
DIRECTIONS:
Mix 3-5 gallons of HB Zinc Supreme with 50 gallons of clean, warm water to create final solution for use in footbaths for cattle.For best results:
Cattle hooves need to be clean prior to walking through the footbath solution to allow complete and effective contact with the hoof. If conditions exist that cause hooves to be dirty a prewash or rinse bath with Hoof Bath Detergent hoof cleaner conditioner should be used in front of the treatment footbath.Solution should be changed after 200 cow passes.
Use in conjunction with a total hoof care program including proper hoof trimming, attention to cow comfort and sanitation, correct nutrition and proper footbath management.
Footbath treatments are recommended every other day at a minimum as a good management practice for maintaining healthy hooves.
It is recommended that you switch out active footbath treatment every several weeks to avoid overuse of a single active ingredient.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HB ZINC SUPREME
zinc footbath solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:64892-024 Route of Administration Topical Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC SULFATE MONOHYDRATE (UNII: PTX099XSF1) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 339.1 g in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64892-024-16 18.9 L in 1 PAIL 2 NDC:64892-024-17 56.78 L in 1 DRUM 3 NDC:64892-024-18 113.6 L in 1 DRUM 4 NDC:64892-024-21 208.2 L in 1 DRUM 5 NDC:64892-024-88 1040 L in 1 CONTAINER, FLEXIBLE INTERMEDIATE BULK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/07/2020 Labeler - Wausau Chemical (006136220) Registrant - Wausau Chemical (006136220) Establishment Name Address ID/FEI Business Operations Wausau Chemical 006136220 manufacture, api manufacture