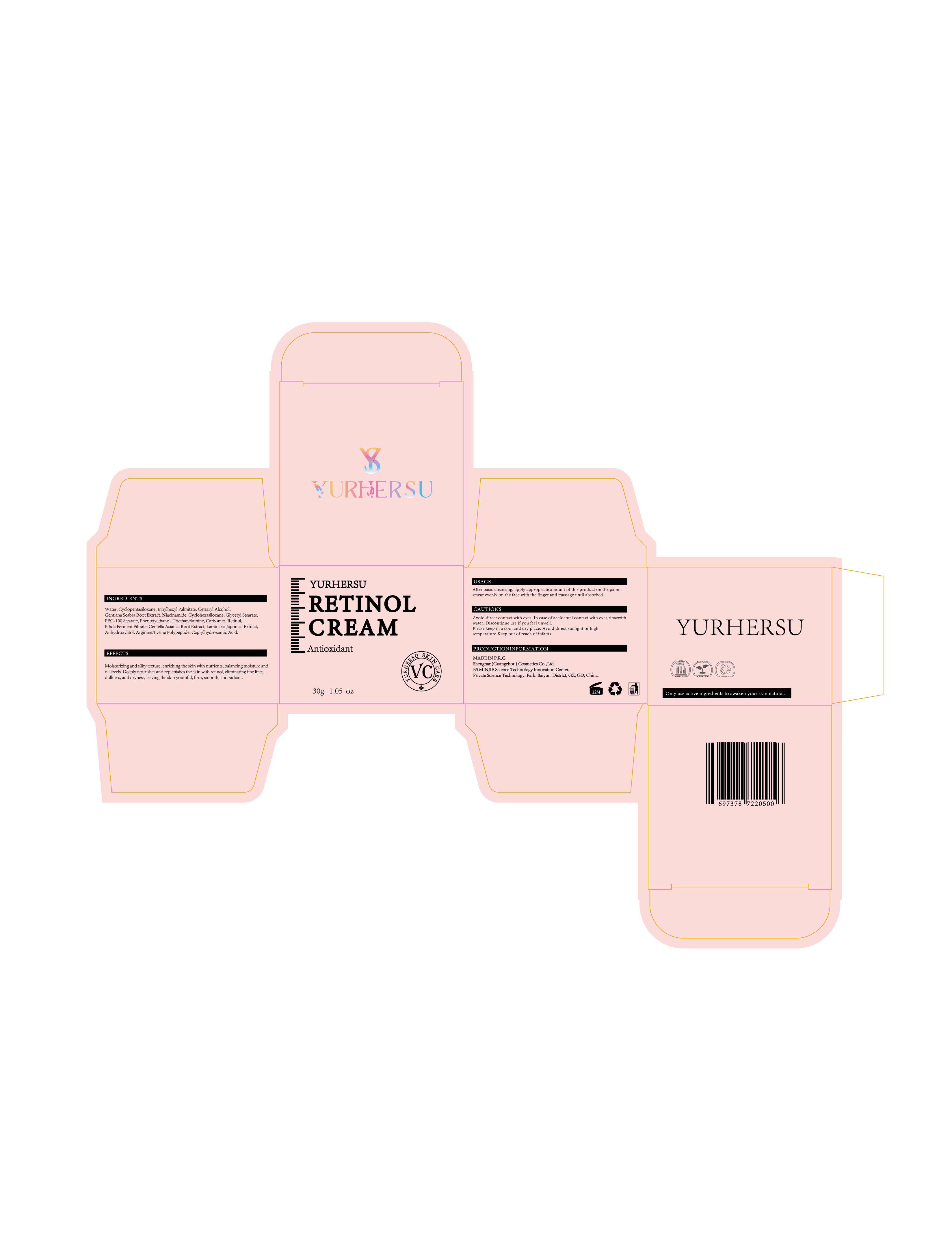
Label: RERINOLCREAM- retinol cream
- NDC Code(s): 84019-011-01
- Packager: Shengnan (Guangzhou) Cosmetics Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS
- PURPOSE
- STOP USE
- ACTIVE INGREDIENT
- WHEN USING
-
INACTIVE INGREDIENT
Water,Cyclopentasiloxane,Ethylhexyl Palmitate,Cetearyl Alcohol,Gentiana Scabra Root Extract,Niacinamide,Cyclohexasiloxane,Glyceryl Stearate,PEG-100 Stearate,Phenoxyethanol,Triethanolamine,Carbomer,Bifida Ferment Filtrate,Centella Asiatica Root Extract,Laminaria Japonica Extract,Anhydroxylitol,Arginine/Lysine Polypeptide,Caprylhydroxamic Acid
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RERINOLCREAM
retinol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84019-011 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RETINOL (UNII: G2SH0XKK91) (RETINOL - UNII:G2SH0XKK91) RETINOL 100 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOPENTASILOXANE (UNII: 0THT5PCI0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84019-011-01 30 g in 1 BOTTLE; Type 0: Not a Combination Product 11/23/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 11/23/2024 Labeler - Shengnan (Guangzhou) Cosmetics Co., Ltd (541200425) Establishment Name Address ID/FEI Business Operations Shengnan (Guangzhou) Cosmetics Co., Ltd 541200425 manufacture(84019-011)