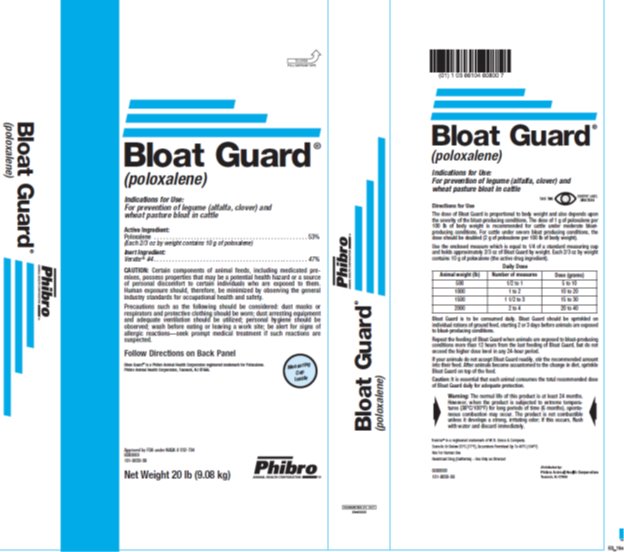
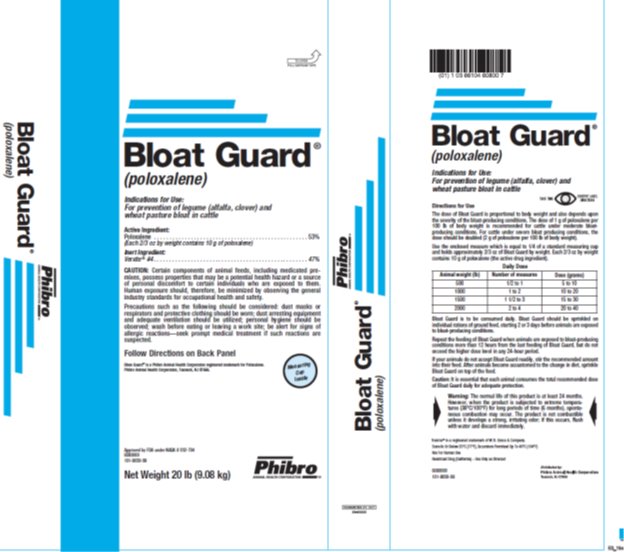
Label: BLOAT GUARD- poloxalene powder
- NDC Code(s): 66104-6080-0
- Packager: Phibro Animal Health
- Category: OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated January 27, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
-
CAUTION:
Certain components of animal feeds, including medicated premixes, possess properties that may be a potential health hazard or a source of personal discomfort to certain individuals who are exposed to them. Human exposure should, therefore, be minimized by observing the general industry standards for occupational health and safety.
Precautions such as the following should be considered: dust masks or respirators and protective clothing should be worn; dust arresting equipment and adequate ventilation should be utilized; personal hygiene should be observed; wash before eating or leaving a work site; be alert for signs of allergic reactions—seek prompt medical treatment if such reactions are suspected.
Follow Directions on Back Panel
Bloat Guard® is a Phibro Animal Health Corporation registered trademark for Poloxalene.
Phibro Animal Health Corporation, Teaneck, NJ 07666.
Approved by FDA under NADA #032-704
6080000
101-9030-09
-
Directions for Use The dose of Bloat Guard is proportional to body weight and also depends upon the severity of the bloat-producing conditions. The dose of 1 g of poloxalene per 100 lb of body weight is recommended for cattle under moderate bloat-producing conditions. For cattle under severe bloat producing conditions, the dose should be doubled (2 g of poloxalene per 100 lb of body weight).Use the enclosed measure which is equal to 1/4 of a standard measuring cup and holds approximately 2/3 oz of Bloat Guard by weight. Each 2/3 oz by weight contains 10 g of poloxalene (the active drug ingredient).Daily DoseAnimal WeightNumber of measuresDose (grams)500½ to 15 to 1010001 to 210 to 2015001 ½ to 315 to 3020002 to 420 to 40
Bloat Guard is to be consumed daily. Bloat Guard should be sprinkled on individual rations of ground feed, starting 2 or 3 days before animals are exposed to bloat-producing conditions.
Repeat the feeding of Bloat Guard when animals are exposed to bloat-producing conditions more than 12 hours from the last feeding of Bloat Guard, but do not exceed the higher dosage levels in any 24-hour period.
If your animals do not accept Bloat Guard readily, stir the recommended amount into their feed. After animals become accustomed to the change in diet, sprinkle Bloat Guard on top of the feed.
Caution: It is essential that each animal consumes the total recommended dosage of Bloat Guard daily for adequate protection.
-
Warning:
The normal life of this product is at least 24 months. However, when the product is subjected to extreme temperatures (38°C/100°F) for long periods of time (6 months), spontaneous combustion may occur. The product is not combustible unless it develops a strong, irritating odor; if this occurs, flush with water and discard immediately.
-
SPL UNCLASSIFIED SECTION
Verxite® is a registered trademark of W.R. Grace & Company.
Store At or Below 25°C (77°F), Excursions Permitted Up To 40°C (104°F)
NOT FOR HUMAN USE
Restricted Drug (California) – Use Only As Directed
Bloat Guard® is a Phibro Animal Health registered trademark for Poloxalene
Distributed by:
Phibro Animal Health Corporation, Teaneck, NJ 07666.
6080000
101-9030-09
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BLOAT GUARD
poloxalene powderProduct Information Product Type OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG Item Code (Source) NDC:66104-6080 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLOXALENE (UNII: V8B3K56SW0) (POLOXALENE - UNII:V8B3K56SW0) POLOXALENE 240.4 g in 0.45 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66104-6080-0 9.08 kg in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA032704 03/10/1966 Labeler - Phibro Animal Health (006989008) Registrant - Phibro Animal Health (006989008)