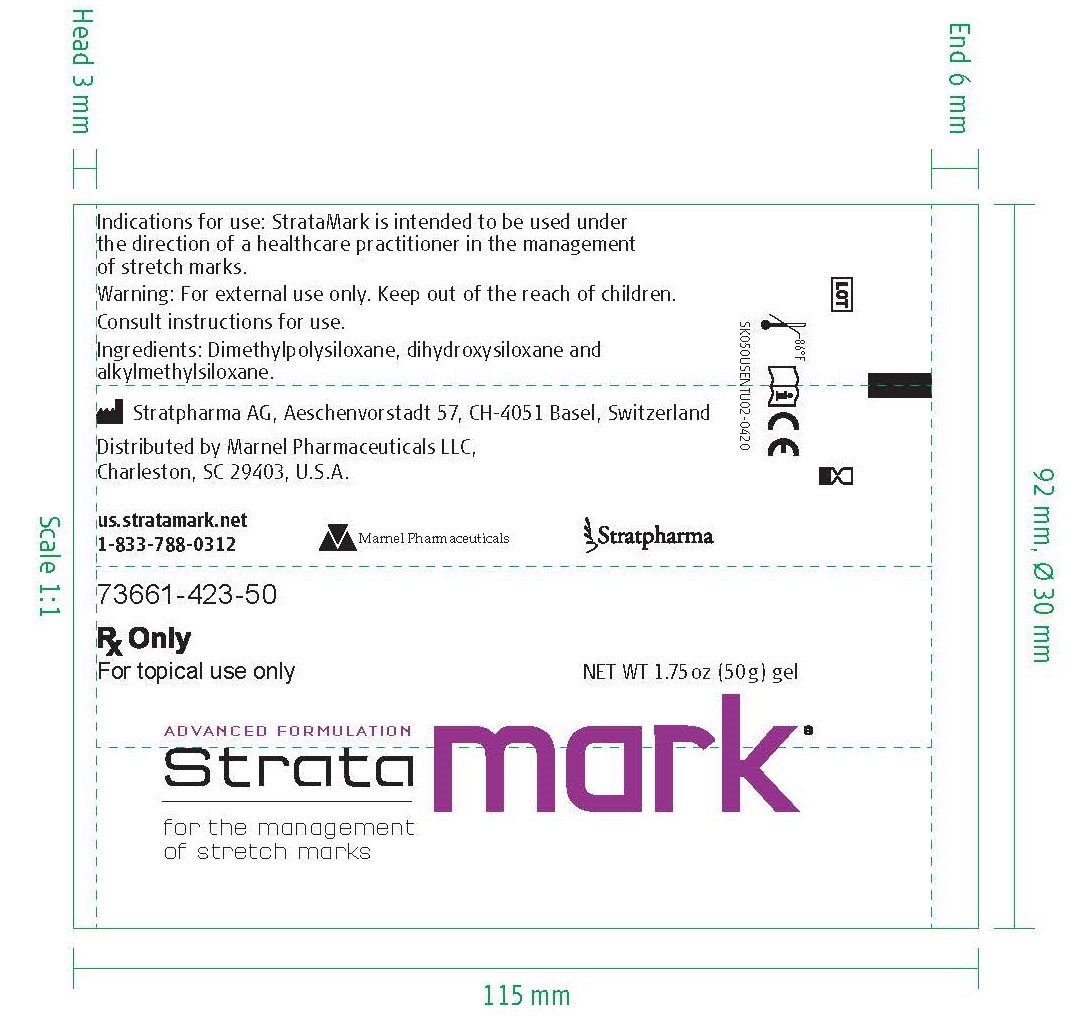
Label: STRATAMARK-
- NHRIC Code(s): 73661-423-50
- Packager: Marnel Pharmaceuticals, Inc.
- Category: MEDICAL DEVICE
- DEA Schedule: None
- Marketing Status: Exempt device
Drug Label Information
Updated July 11, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS AND PRECAUTIONS
-
DESCRIPTION
[EN]Description
StrataMark is a rapidly drying, non-sticky, transparent gel formulation for the management of Striae Distensae (stretch marks).
When used as directed, StrataMark dries to form a thin, flexible and protective layer, that is gas permeable and water-proof which hydrates and protects stretch marks, allowing the skin to normalize the collagen synthesis cycle.
StrataMark is suitable for all areas of the skin, including exposed and sensitive areas, as well as large areas of the body (i.e. buttocks, abdomen, thighs, breasts and back).
-
INDICATIONS & USAGE
Indication for use
StrataMark is intended to be used under the direction of healthcare practitioners in the management of stretch marks.
StrataMark is indicated for the management of all types of stretch marks, particularly those covering a wide surface area, resulting from pregnancy, adolescent growth spurts, weight gain or loss, or bodybuilding.
StrataMark is indicated to soften and flatten stretch marks, reduce redness and discoloration associated with stretch marks as well as to relieve the itching and discomfort of stretch marks.
It contains no alcohols, parabens or fragrances.
StrataMark is suitable for pregnant women, breastfeeding mothers, children and people with sensitive skin.
StrataMark is intended for single patient use.
-
INDICATIONS & USAGE
Directions for use
On the first use of 1.75 oz (50g) tubes, remove the cap, then the protective seal and close with the cap after use.
Ensure that the skin is clean and dry.
Apply a very thin layer of StrataMark to the relevant areas with the fingertips and distribute evenly to form a very thin layer and allow the gel to dry.
When applied, StrataMark should be dry in 5-6 minutes.
If it takes longer to dry you have probably applied too much. Gently remove the excess with a clean tissue or gauze and allow the drying process to continue.
Once dry, StrataMark can be covered with undergarments, sunscreens or cosmetics.
StrataMark should be applied once per day or as advised by your physician.
For best results StrataMark should be maintained in continuous contact with the skin (24 hours a day/7 days a week).
- INFORMATION FOR PATIENTS
-
WARNINGS
Warning
- For external use only.
- StrataMark should not be applied to third degree burns or to open wounds.
- StrataMark should not be placed in contact with eyes.
- StrataMark should not be applied over other skin treatments without the advice of your physician.
- StrataMark may stain clothing if not completely dry. If staining occurs, dry cleaning should be able to remove it without damaging the fabric.
- For correct storage please reclose the tube tightly with the cap.
- If irritation occurs, discontinue use and consult your physician.
- Keep out of the reach of children.
- Do not use after the expiration (EXP) date printed on the tube. The expiration (EXP) date does not change once the tube has been opened.
- Do not use if the tube is damaged.
- CONTRAINDICATIONS
- SPL UNCLASSIFIED SECTION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STRATAMARK
dressing, wound, occlusiveProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:73661-423 Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) DIMETHICONOL (2000 CST) (UNII: T74O12AN6Y) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:73661-423-50 1 in 1 BOX 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date exempt device NAD 07/11/2020 Labeler - Marnel Pharmaceuticals, Inc. (080161449) Establishment Name Address ID/FEI Business Operations Stratpharma AG 483232695 manufacture