Label: PRINZING EYE WASH STATION- kit
- NHRIC Code(s): 55925-100-02
- NDC Code(s): 64809-101-16
- Packager: BRADY MEXICO, S. DE R.L. DE C.V.
- Category: MEDICAL DEVICE
- DEA Schedule: None
- Marketing Status: Exempt device
Drug Label Information
Updated June 24, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
PRINZING Part #DSP EW1
PRINZING
Part #DSP EW1
THIS BOX CONTAINS
NO. DESCRIPTION COUNTRY OF ORIGIN QTY.
Y235674 32 OZ. BOTTLE EYESALINE REFILL USA 2
Y382769 POLY BAGS 16X20 2 MIL CHINA 1
Y389397 PRINZING EYE WASH DISPENSER UNBOXED MEXICO 1
Y526795 INSERT A FOR Y391541 USA 2
Y526796 INSERT B FOR Y391541 USA 2
Y526794 CARTON BOX (CAJA) FOR Y391541 USA 1
Y395186 PKG LABEL .B424 3.0"X2.9", MEX/GH MEXICO 1
Y34465 THT-25-424-1 MEXICO 2
WO: 23128444
*Y384842*
BRADY MEXICO S DE RL DE CV
Guerrero Negro No. 2, NORDIKA,
Tijuana, Baja California,
Mexico, C.P. 22644
PACKAGED IN MEXICO
PRINZING
PRODUCT: Prinzing Eyewash Station Equipped
MANUFACTURED BY: BRADY MEXICO S DE RL DE CV
Guerrero Negro No. 2, NORDIKA,
Tijuana, Baja California, Mexico, C.P. 22644
CONTENT: One Eye Wash Station
DIRECTIONS FOR USE
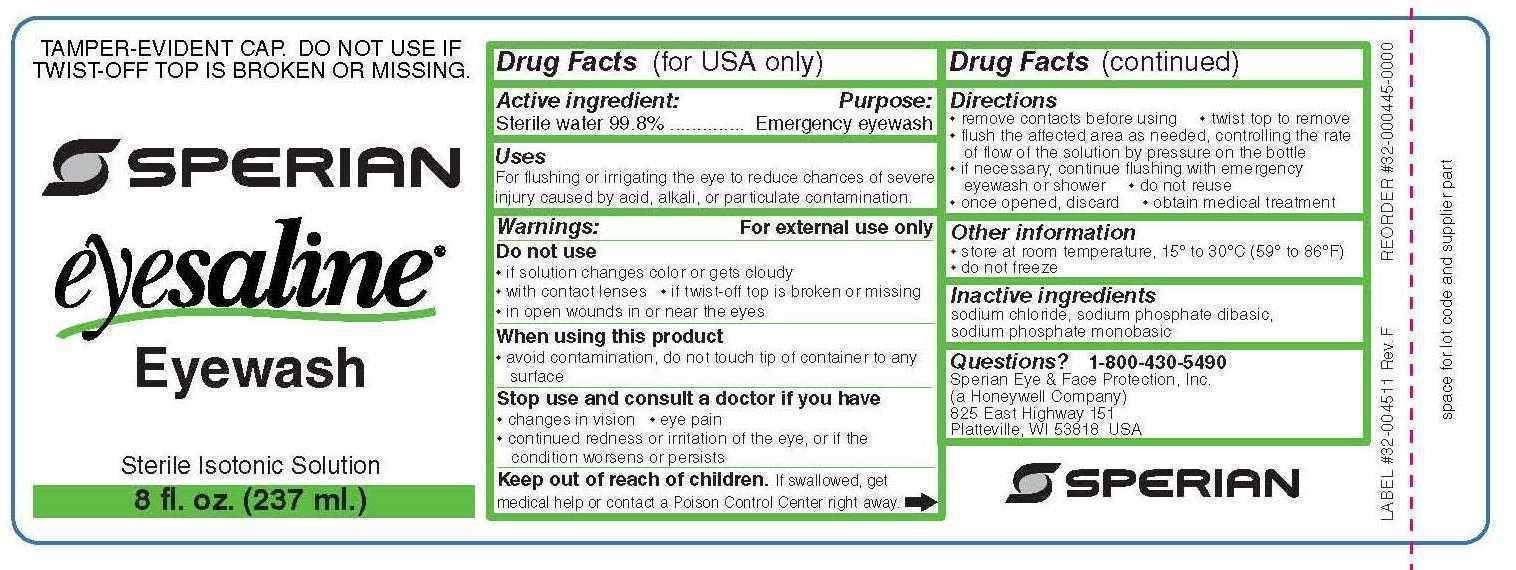
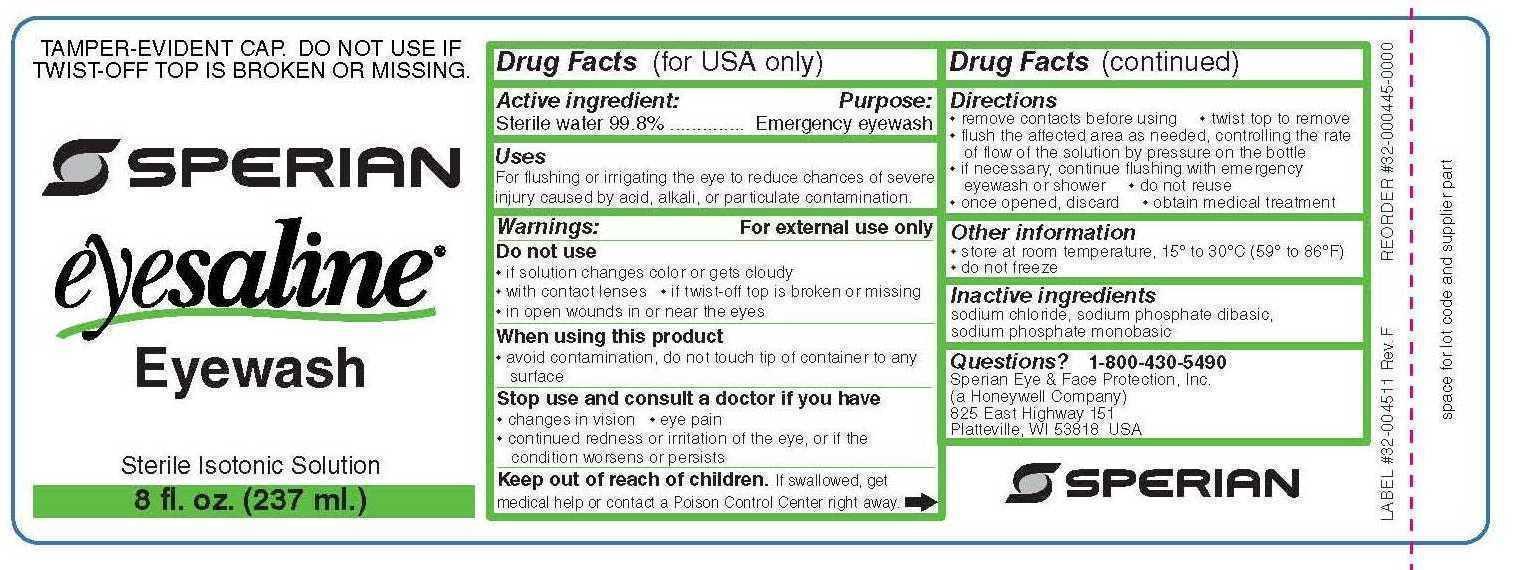
Purposes: emergency eyewash and skin flush.
Use for flushing the eye to reduce chances of severe injury caused by acid, alkali, or particulate contamination.
Directions for use: - twist cap open - do not dilute solution or reuse bottle - hold container a few inches above the eye or skin - control flow of solution by pressure on bottle - flush affected area as needed a minimum of 20 minutes
Warnings: for external use only
Do not use: - for injection - intraocular surgery - if solution changes color or becomes cloudy
When using this product: - avoid contamination, do not touch tip of container to any surface - do not reuse - once opened, discard - obtain immediate medical treatment for all open wounds in or near the eyes
Ask doctor if you have: - eye pain - changes in vision - redness or irritation of the eye after use - an injury cause by alkali - condition worsens or persists
Keep out of reach of children - If swallowed, get medical help or contact a poision control center right away
Other information: - tamper evident: do not use if twist off top is broken or missing - not for use as a contact lens solution - use before expiration date marked on bottle - store at room temperature 15 degrees Celcius to 30 degrees Celcius (59 degrees - 86 degrees Fahrenheit)

- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PRINZING EYE WASH STATION
first aid kit with drug kitProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:55925-100 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:55925-100-02 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 946 mL Part 1 of 1 SPERIAN EYESALINE EYEWASH
water liquidProduct Information Item Code (Source) NDC:64809-101 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 99.8 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM PHOSPHATE, DIBASIC ANHYDROUS (UNII: 22ADO53M6F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64809-101-16 1892 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 09/07/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date exempt device ABC 07/02/2013 Labeler - BRADY MEXICO, S. DE R.L. DE C.V. (812761655) Establishment Name Address ID/FEI Business Operations Sperian Eye & Face Protection Inc 013435034 manufacture, label