Label: REVALOR-XS- trenbolone acetate and estradiol implant
- NDC Code(s): 57926-026-01
- Packager: Merck Sharp & Dohme Corp.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated April 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Revalor®-XS is an extended-release implant. One dose (implant) contains 200 mg of trenbolone acetate and 40 mg of estradiol in 6 coated and 4 uncoated pellets, each containing 20 mg trenbolone acetate and 4 mg estradiol.
The small yellow pellets are coated with a polymer to provide extended release of the active ingredients. One cartridge contains 10 doses.
Manufactured by a non-sterilizing process.
-
INDICATIONS FOR USE
For increased rate of weight gain and improved feed efficiency for up to 200 days after implantation in growing beef steers fed in confinement for slaughter.
Do not use in beef calves less than 2 months of age, dairy calves, and veal calves because effectiveness and safety have not been established.
Do not use in animals intended for subsequent breeding, or in dairy cows.
-
DIRECTIONS
The cartridge is designed to be used with a special implanting tool. The special implanting tool is available from Intervet Inc. Ten doses (implants) are in each cartridge. The implant is placed under the skin on the posterior aspect of the ear (see SITE OF IMPLANTATION and METHOD OF USE sections).
With the animal suitably restrained, the skin on the outer surface of the ear should be cleaned. The implant is then administered by the method shown in the diagram below.
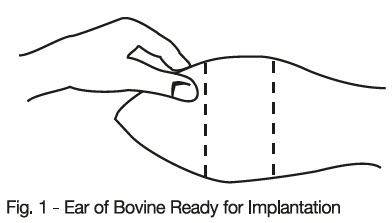
-
SITE OF IMPLANTATION
After appropriately restraining the animal to allow access to the ear, cleanse the skin at the implant needle puncture site. It is subcutaneous between the skin and cartilage on the back side of the ear and below the midline of the ear. The implant must not be placed closer to the head than the edge of the cartilage ring farthest from the head. The location of insertion of the needle is a point toward the tip of the ear and at least a needle length away from the intended deposition site. Care should be taken to avoid injuring the major blood vessels or cartilage of the ear.
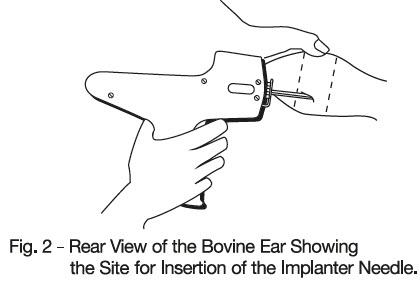
-
METHOD OF USE
- Do not remove the cap of the cartridge containing the implants.
- Place the cartridge (D) with the capped end to the front into slot at the top of the implanter magazine marked (A) on the diagram.
- Gently push the cartridge into the slot until it clicks into place.
- The implanter is then ready for use.
- Take the ear of the animal firmly with the free hand in the manner shown in Fig. 1. Then insert the needle into the subcutaneous tissue at the point indicated in Fig. 2.
- After inserting the needle to its full extent, squeeze the trigger (E) gradually. Allow the pellets of the implant to be deposited in a single row.
- Withdraw the implanter. This will advance the cartridge one groove in the magazine and the next implant is now ready for use.
- When all the implants have been administered, the cartridge will discharge out the bottom of the magazine and may be replaced by a new one.
- To change the needle, loosen the needle locking nut labeled (F) in Fig. 3 and replace the needle. Tighten the nut finger tight and the implanter is ready for use.
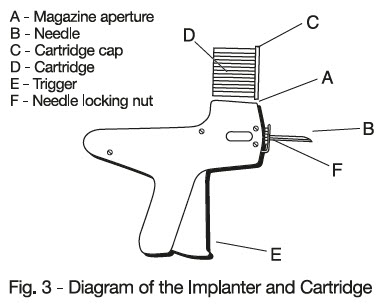
-
WITHDRAWAL PERIODS AND RESIDUE WARNINGS:
No withdrawal period is required when used according to labeling.
Do not use in beef calves less than 2 months of age, dairy calves, and veal calves. A withdrawal period has not been established for this product in pre-ruminating calves.
Do not use in dairy cows or in animals intended for subsequent breeding. Use in these cattle may cause drug residues in milk and/or in calves born to these cows.
Implant pellets subcutaneously in ear only. Any other location is a violation of Federal law. Do not attempt salvage of implanted site for human or animal food.
- USER SAFETY WARNINGS
- STORAGE CONDITIONS
- HOW SUPPLIED
-
SPL UNCLASSIFIED SECTION
Made in Austria by:
Intervet GesmbHDistributed by:
Intervet Inc (d/b/a Merck Animal Health)
Madison, NJ 07940Restricted Drug (California) - Use only as Directed
Approved by FDA under NADA # 141-269
US Patent # RE 39,592 E
Revalor is a registered trademark of Intervet Inc. or an affiliate.Rev. 05/2022
384297 R5 - PRINCIPAL DISPLAY PANEL - 10 x 10 Cartridge Implant Box
-
INGREDIENTS AND APPEARANCE
REVALOR-XS
trenbolone acetate and estradiol implantProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:57926-026 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRENBOLONE ACETATE (UNII: RUD5Y4SV0S) (TRENBOLONE - UNII:P53R4420TR) TRENBOLONE ACETATE 200 mg ESTRADIOL (UNII: 4TI98Z838E) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL 40 mg Inactive Ingredients Ingredient Name Strength CHOLESTEROL (UNII: 97C5T2UQ7J) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) MAGNESIUM STEARATE (UNII: 70097M6I30) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57926-026-01 10 in 1 BOX 1 10 in 1 CARTRIDGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141269 01/19/2007 Labeler - Merck Sharp & Dohme Corp. (001317601) Establishment Name Address ID/FEI Business Operations Intervet GESMBH 303380992 MANUFACTURE Establishment Name Address ID/FEI Business Operations Euroapi France 276495414 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Aspen Oss b.v. 491013870 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Valdepharm 260128560 API MANUFACTURE


