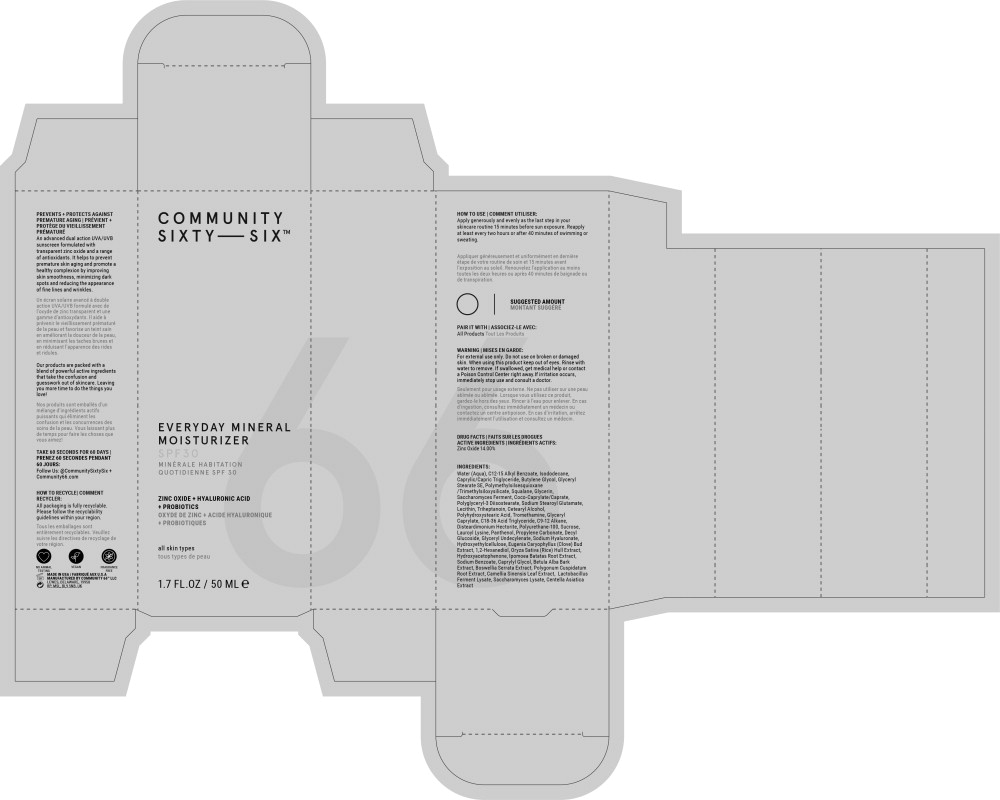
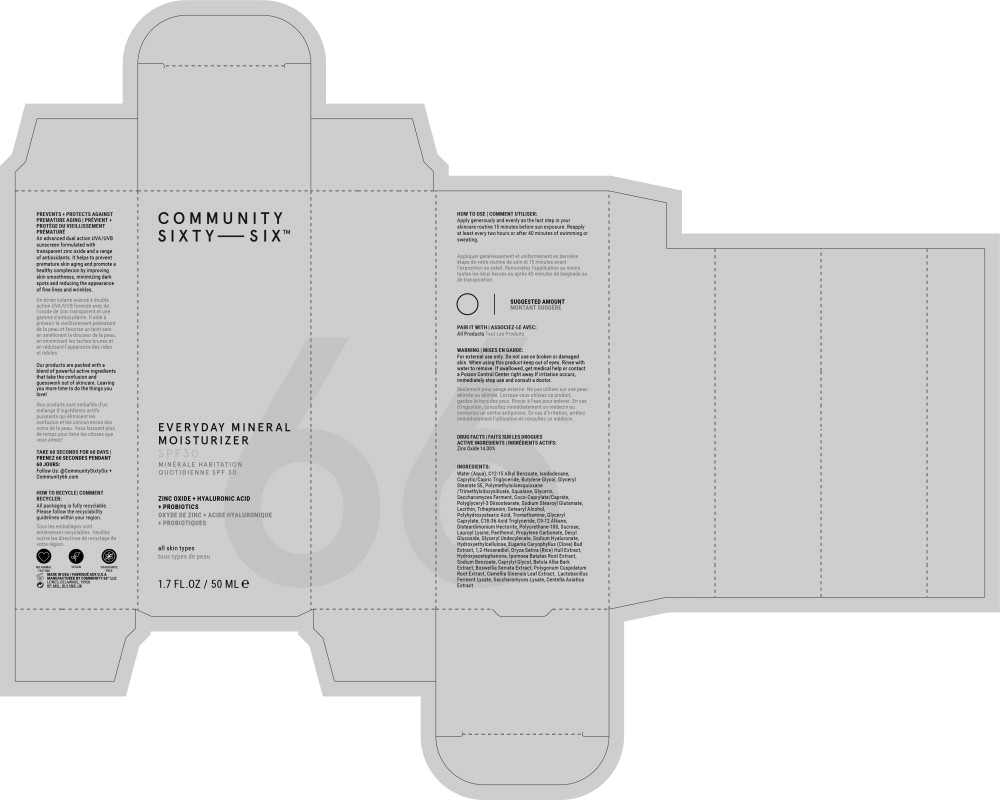
Label: EVERYDAY MINERAL MOISTURIZER SPF 30- zinc oxide lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 82769-100-10, 82769-100-12 - Packager: Community 66, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 28, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HOW TO USE | COMMENT UTILISER:
Apply generously and evenly as the last step in your skincare routine 15 minutes before sun exposure. Reapply at least every two hours or after 40 minutes of swimming or sweating.
Appliquer généreusement et uniformément en dernière étape de votre routine de soin et 15 minutes avant l'exposition au soleil. Renouvelez l'application au moins toutes les deux heures ou après 40 minutes de baignade ou de transpiration.
SUGGESTED AMOUNT
MONTANT SUGGÉRÉ
- PAIR IT WITH | ASSOCIEZ-LE AVEC:
-
WARNING | MISES EN GARDE:
For external use only.
When using this product keep out of eyes. Rinse with water to remove. If swallowed, get medical help or contact a Poison Control Center right away. If irritation occurs, immediately stop use and consult a doctor.
Seulement pour usage externe. Ne pas utiliser sur une peau abîmée ou abîmée. Lorsque vous utilisez ce produit, gardez-le hors des yeux. Rincer à l'eau pour enlever. En cas d'ingestion, consultez immédiatement un médecin ou contactez un centre antipoison. En cas d'irritation, arrêtez immédiatement l'utilisation et consultez un médecin.
DRUG FACTS | FAITS SUR LES DROGUES
- ACTIVE INGREDIENTS | INGRÉDIENTS ACTIFS:
-
INGREDIENTS:
Water (Aqua), C12-15 Alkyl Benzoate, Isododecane, Caprylic/Capric Triglyceride, Butylene Glycol, Glyceryl Stearate SE, Polymethylsilsesquioxane /Trimethylsiloxysilicate, Squalane, Glycerin, Saccharomyces Ferment, Coco-Caprylate/Caprate, Polyglyceryl-3 Diisostearate, Sodium Stearoyl Glutamate, Lecithin, Triheptanoin, Cetearyl Alcohol, Polyhydroxystearic Acid, Tromethamine, Glyceryl Caprylate, C18-36 Acid Triglyceride, C9-12 Alkane, Disteardimonium Hectorite, Polyurethane-100, Sucrose, Lauroyl Lysine, Panthenol, Propylene Carbonate, Decyl Glucoside, Glyceryl Undecylenate, Sodium Hyaluronate, Hydroxyethylcellulose, Eugenia Caryophyllus (Clove) Bud Extract, 1,2-Hexanediol, Oryza Sativa (Rice) Hull Extract, Hydroxyacetophenone, Ipomoea Batatas Root Extract, Sodium Benzoate, Caprylyl Glycol, Betula Alba Bark Extract, Boswellia Serrata Extract, Polygonum Cuspidatum Root Extract, Camellia Sinensis Leaf Extract, Lactobacillus Ferment Lysate, Saccharomyces Lysate, Centella Asiatica Extract
- Principal Display Panel – 50 mL Carton Label
- Principal Display Panel – 50 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
EVERYDAY MINERAL MOISTURIZER SPF 30
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82769-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 136 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Isododecane (UNII: A8289P68Y2) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Butylene Glycol (UNII: 3XUS85K0RA) Glyceryl Stearate SE (UNII: FCZ5MH785I) Polymethylsilsesquioxane/Trimethylsiloxysilicate (UNII: X2PZH4Y6HT) Squalane (UNII: GW89575KF9) Glycerin (UNII: PDC6A3C0OX) Cocoyl Caprylocaprate (UNII: 8D9H4QU99H) Polyglyceryl-3 Diisostearate (UNII: 46P231IQV8) Sodium Stearoyl Glutamate (UNII: 65A9F4P024) Lecithin, Soybean (UNII: 1DI56QDM62) Triheptanoin (UNII: 2P6O7CFW5K) Cetostearyl Alcohol (UNII: 2DMT128M1S) Polyhydroxystearic Acid (2300 MW) (UNII: YXH47AOU0F) Tromethamine (UNII: 023C2WHX2V) Glyceryl Monocaprylate (UNII: TM2TZD4G4A) C18-36 Acid Triglyceride (UNII: ZRA72DR3R7) Disteardimonium Hectorite (UNII: X687XDK09L) Sucrose (UNII: C151H8M554) Lauroyl Lysine (UNII: 113171Q70B) Panthenol (UNII: WV9CM0O67Z) Polysorbate 20 (UNII: 7T1F30V5YH) Propylene Carbonate (UNII: 8D08K3S51E) Decyl Glucoside (UNII: Z17H97EA6Y) Glyceryl 1-Undecylenate (UNII: B68LJT9544) Hyaluronate Sodium (UNII: YSE9PPT4TH) Hydroxyethyl Cellulose, Unspecified (UNII: T4V6TWG28D) Clove (UNII: K48IKT5321) 1,2-Hexanediol (UNII: TR046Y3K1G) Rice Germ (UNII: 7N2B70SFEZ) Hydroxyacetophenone (UNII: G1L3HT4CMH) Sweet Potato (UNII: M9WGG9Z9GK) Sodium Benzoate (UNII: OJ245FE5EU) Caprylyl Glycol (UNII: 00YIU5438U) Betula Pubescens Bark (UNII: 3R504894L9) Indian Frankincense (UNII: 4PW41QCO2M) Reynoutria Japonica Leaf (UNII: 2540B7G25G) Green Tea Leaf (UNII: W2ZU1RY8B0) Sodium Phosphate, Dibasic, Anhydrous (UNII: 22ADO53M6F) Polysorbate 60 (UNII: CAL22UVI4M) Saccharomyces Lysate (UNII: R85W246Z1C) Centella Asiatica Whole (UNII: 7M867G6T1U) Citric Acid Monohydrate (UNII: 2968PHW8QP) Sodium Phosphate (UNII: SE337SVY37) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82769-100-10 1 in 1 BOX 07/05/2022 1 NDC:82769-100-12 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 07/05/2022 Labeler - Community 66, LLC (118547190)