Label: SIMETHICONE- dimethicone capsule, liquid filled
-
Contains inactivated NDC Code(s)
NDC Code(s): 68210-1902-1, 68210-1902-2, 68210-1902-3, 68210-1902-4, view more68210-1902-5 - Packager: SPIRIT PHARMACEUTICALS,LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 2, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (per softgel)
- Purpose
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
-
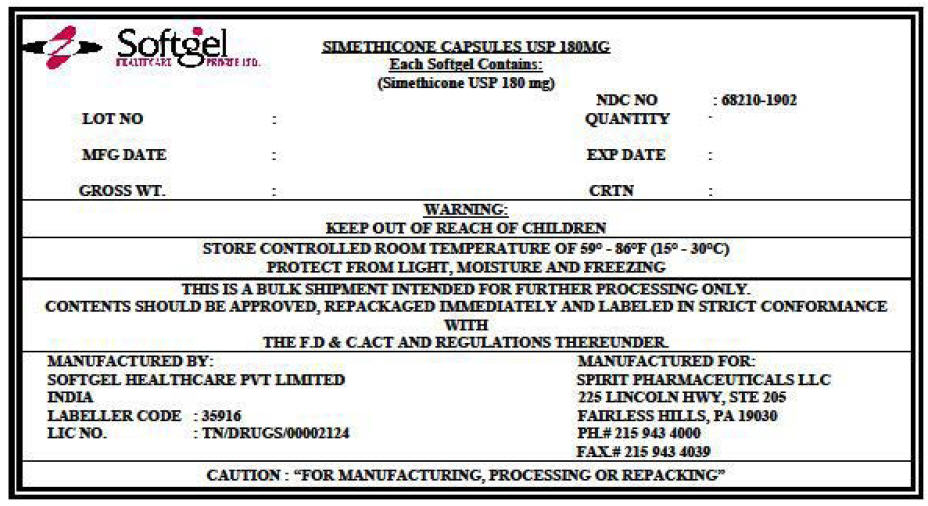
PRINCIPAL DISPLAY PANEL - Shipping Label
SIMETHICONE CAPSULES USP 180MG
Each Softgel Contains:
(Simethicone USP 180 mg)LOT NO :
MFG DATE :
GROSS WT. :
NDC NO : 68210-1902
QUANTITY :EXP DATE :
CRTN :
WARNING:
KEEP OUT OF REACH OF CHILDRENSTORE CONTROLLED ROOM TEMPERATURE OF 59° - 86°F (15° - 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZINGTHIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED, REPACKAGED IMMEDIATELY AND LABELED IN STRICT CONFORMANCE
WITH
THE F.D & C.ACT AND REGULATIONS THEREUNDER.MANUFACTURED BY:
SOFTGEL HEALTHCARE PVT LIMITED
INDIA
LABELLER CODE : 35916
LIC NO. : TN/DRUGS/00002124MANUFACTURED FOR:
SPIRIT PHARMACEUTICALS LLC
225 LINCOLN HWY, STE 205
FAIRLESS HILLS, PA 19030
PH.# 215 943 4000
FAX.# 215 943 4039CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"
-
INGREDIENTS AND APPEARANCE
SIMETHICONE
dimethicone capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68210-1902 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 180 mg Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) HYPROMELLOSE (UNII: 3NXW29V3WO) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) PEPPERMINT OIL (UNII: AV092KU4JH) Product Characteristics Color ORANGE Score no score Shape OVAL Size 10mm Flavor Imprint Code P10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68210-1902-1 1 in 1 BOX 1 10 in 1 BAG 2 NDC:68210-1902-2 1 in 1 BOX 2 30 in 1 BAG 3 NDC:68210-1902-3 1 in 1 BOX 3 1000 in 1 BAG 4 NDC:68210-1902-4 1 in 1 BOX 4 16000 in 1 BAG 5 NDC:68210-1902-5 1 in 1 BOX 5 20000 in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part310.545 10/01/2009 Labeler - SPIRIT PHARMACEUTICALS,LLC (179621011) Establishment Name Address ID/FEI Business Operations SOFTGEL HEALTHCARE PVT LTD 675584180 MANUFACTURE
