Label: CEFADROXIL powder, for suspension
-
NDC Code(s):
68180-181-01,
68180-181-02,
68180-182-01,
68180-182-02, view more68180-182-03
- Packager: Lupin Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated October 31, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
Rx only
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefadroxil for oral suspension and other antibacterial drugs, cefadroxil for oral suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
-
DESCRIPTION
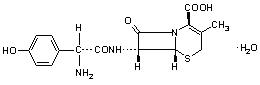
Cefadroxil monohydrate is a semisynthetic cephalosporin antibiotic intended for oral administration. It is a white to yellowish-white crystalline powder. It is soluble in water and it is acid-stable. It is chemically designated as 5-Thia-1-azabicyclo[4.2.O]oct-2-ene-2-carboxylic acid, 7-[[amino(4-hydroxyphenyl)acetyl]amino]-3-methyl-8-oxo-, monohydrate, [6R-[6(,7((R*)]]-. It has the formula C16H17N3O5S•H2O and the molecular weight of 381.40. It has the following structural formula:
Cefadroxil for oral suspension USP contains cefadroxil monohydrate USP. After reconstitution, each 5 mL contains cefadroxil monohydrate USP equivalent to 250 mg or 500 mg of cefadroxil. In addition, cefadroxil for oral suspension USP contains the following inactive ingredients: colloidal silicon dioxide, FD&C Yellow No. 6, powder flavor orange, powder flavor pineapple, sodium benzoate, sucrose, and xanthan gum.
Cefadroxil for oral suspension USP is a light orange colored powder, forming orange colored suspension on constitution.
-
CLINICAL PHARMACOLOGY
Cefadroxil monohydrate is rapidly absorbed after oral administration. Following single doses of 500 mg and 1000 mg, average peak serum concentrations were approximately 16 and 28 mcg/mL, respectively. Measurable levels were present 12 hours after administration. Over 90% of the drug is excreted unchanged in the urine within 24 hours. Peak urine concentrations are approximately 1800 mcg/mL during the period following a single 500-mg oral dose. Increases in dosage generally produce a proportionate increase in cefadroxil monohydrate urinary concentration. The urine antibiotic concentration, following a 1-g dose, was maintained well above the MIC of susceptible urinary pathogens for 20 to 22 hours.
Microbiology
In vitro tests demonstrate that the cephalosporins are bactericidal because of their inhibition of cell-wall synthesis. Cefadroxil has been shown to be active against the following organisms both in vitro and in clinical infections (see INDICATIONS AND USAGE):
Beta-hemolytic streptococci
Staphylococci, including penicillinase-producing strains
Streptococcus (Diplococcus) pneumoniae
Escherichia coli
Proteus mirabilis
Klebsiella species
Moraxella (Branhamella) catarrhalis
Note: Most strains of Enterococcus faecalis (formerly Streptococcus faecalis) and Enterococcus faecium (formerly Streptococcus faecium) are resistant to cefadroxil monohydrate. It is not active against most strains of Enterobacter species, Morganella morganii (formerly Proteus morganii), and P. vulgaris. It has no activity against Pseudomonas species and Acinetobacter calcoaceticus (formerly Mima and Herellea species).
Susceptibility Tests
Diffusion Techniques:
The use of antibiotic disk susceptibility test methods which measure zone diameter give an accurate estimation of antibiotic susceptibility. One such standard procedure1 which has been recommended for use with disks to test susceptibility of organisms to cefadroxil uses the cephalosporin class (cephalothin) disk. Interpretation involves the correlation of the diameters obtained in the disk test with the minimum inhibitory concentration (MIC) for cefadroxil.
Reports from the laboratory giving results of the standard single-disk susceptibility test with a 30 mcg cephalothin disk should be interpreted according to the following criteria:
Interpretive Criteria for Enterobacteriaceae, and Staphylococcus spp. Zone Diameter (mm)
Interpretation
MIC (mcg/mL)
≥18
Susceptible (S)
≤8
15 to 17
Intermediate (I)
-
≤14
Resistant (R)
≥32
A report of "Susceptible" indicates that the pathogen is likely to be inhibited by generally achievable blood levels. A report of "Intermediate susceptibility" suggests that the organism would be susceptible if high dosage is used or if the infection is confined to tissue and fluids (e.g., urine) in which high antibiotic levels are attained. A report of "Resistant'' indicates that achievable concentrations of the antibiotic are unlikely to be inhibitory and other therapy should be selected.
Standardized procedures require the use of laboratory control organisms. The 30 mcg cephalothin disk should give the following zone diameters:
Organism
Zone Diameter (mm)
Staphylococcus aureus ATCC 25923
29 to 37
Escherichia coli ATCC 25922
15 to 21
Dilution Techniques:
When using the CLSI agar dilution or broth dilution (including microdilution) method2,3 or equivalent, the MIC values should be interpreted according to the following criteria:
Interpretive Criteria for Enterobacteriaceae, and Staphylococcus spp. MIC (mcg/mL)
Interpretation
≤8
Susceptible (S)
16
Intermediate (I)
≥32
Resistant (R)
As with standard diffusion methods, dilution procedures require the use of laboratory control organisms. Standard cephalothin powder should provide the following MIC values:
Microorganism
MIC (mcg/mL)
Escherichia coli
ATCC 25922
4 to 16
Staphylococcus aureus
ATCC 29213
0.12 to 0.5
-
INDICATIONS AND USAGE
Cefadroxil for oral suspension USP is indicated for the treatment of patients with infection caused by susceptible strains of the designated organisms in the following diseases:
Urinary tract infections caused by E. coli, P. mirabilis, and Klebsiella species.
Skin and skin structure infections caused by staphylococci and/or streptococci.
Pharyngitis and/or tonsillitis caused by Streptococcus pyogenes (Group A beta-hemolytic streptococci).
Note: Only penicillin by the intramuscular route of administration has been shown to be effective in the prophylaxis of rheumatic fever. Cefadroxil monohydrate is generally effective in the eradication of streptococci from the oropharynx. However, data establishing the efficacy of cefadroxil monohydrate for the prophylaxis of subsequent rheumatic fever are not available.
Note: Culture and susceptibility tests should be initiated prior to and during therapy. Renal function studies should be performed when indicated.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefadroxil for oral suspension and other antibacterial drugs, cefadroxil for oral suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- CONTRAINDICATIONS
-
WARNINGS
BEFORE THERAPY WITH CEFADROXIL MONOHYDRATE IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFADROXIL, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-SENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY.
IF AN ALLERGIC REACTION TO CEFADROXIL MONOHYDRATE OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefadroxil monohydrate, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
PRECAUTIONS
General
Cefadroxil monohydrate should be used with caution in the presence of markedly impaired renal function (creatinine clearance rate of less than 50 mL/min/1.73 m2). (See DOSAGE AND ADMINISTRATION). In patients with known or suspected renal impairment, careful clinical observation and appropriate laboratory studies should be made prior to and during therapy.
Prescribing cefadroxil for oral suspension in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Prolonged use of cefadroxil monohydrate may result in the overgrowth of nonsusceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Cefadroxil monohydrate should be prescribed with caution in individuals with history of gastrointestinal disease particularly colitis.
Information for Patients
Patients should be counseled that antibacterial drugs including cefadroxil for oral suspension should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefadroxil for oral suspension is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefadroxil for oral suspension or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Drug / Laboratory Test Interactions
Positive direct Coombs' tests have been reported during treatment with the cephalosporin antibiotics. In hematologic studies or in transfusion cross-matching procedures when antiglobulin tests are performed on the minor side or in Coombs' testing of newborns whose mothers have received cephalosporin antibiotics before parturition, it should be recognized that a positive Coombs' test may be due to the drug.
Carcinogenesis, Mutagenesis and Impairment of Fertility
No long-term studies have been performed to determine carcinogenic potential. No genetic toxicity tests have been performed.
Teratogenic Effects:
Pregnancy Category B
Reproduction studies have been performed in mice and rats at doses up to 11 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to cefadroxil monohydrate. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Labor and Delivery
Cefadroxil monohydrate has not been studied for use during labor and delivery. Treatment should only be given if clearly needed.
Nursing Mothers
Caution should be exercised when cefadroxil monohydrate is administered to a nursing mother.
Geriatric Use
Of approximately 650 patients who received cefadroxil for the treatment of urinary tract infections in three clinical trials, 28% were 60 years and older, while 16% were 70 years and older. Of approximately 1000 patients who received cefadroxil for the treatment of skin and skin structure infection in 14 clinical trials, 12% were 60 years and older while 4% were 70 years and over. No overall differences in safety were observed between the elderly patients in these studies and younger patients. Clinical studies of cefadroxil for the treatment of pharyngitis or tonsillitis did not include sufficient numbers of patients 65 years and older to determine whether they respond differently from younger patients. Other reported clinical experience with cefadroxil has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Cefadroxil is substantially excreted by the kidney, and dosage adjustment is indicated for patients with renal impairment (see DOSAGE AND ADMINISTRATION: Renal Impairment). Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
Gastrointestinal
Onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment (see WARNINGS). Dyspepsia, nausea and vomiting have been reported rarely. Diarrhea has also occurred.
Hypersensitivity
Allergies (in the form of rash, urticaria, angioedema, and pruritus) have been observed. These reactions usually subsided upon discontinuation of the drug. Anaphylaxis has also been reported.
Other
Other reactions have included hepatic dysfunction including cholestasis and elevations in serum transaminase, genital pruritus, genital moniliasis, vaginitis, moderate transient neutropenia, fever. Agranulocytosis, thrombocytopenia, idiosyncratic hepatic failure, erythema multiforme, Stevens-Johnson syndrome, serum sickness, and arthralgia have been rarely reported.
In addition to the adverse reactions listed above which have been observed in patients treated with cefadroxil, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics:
Toxic epidermal necrolysis, abdominal pain, superinfection, renal dysfunction, toxic nephropathy, aplastic anemia, hemolytic anemia, hemorrhage, prolonged prothrombin time, positive Coombs' test, increased BUN, increased creatinine, elevated alkaline phosphatase, elevated aspartate aminotransferase (AST), elevated alanine aminotransferase (ALT), elevated bilirubin, elevated LDH, eosinophilia, pancytopenia, neutropenia.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment, when the dosage was not reduced (see DOSAGE AND ADMINISTRATION and OVERDOSAGE). If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
-
OVERDOSAGE
A study of children under six years of age suggested that ingestion of less than 250 mg/ kg of cephalosporins is not associated with significant outcomes. No action is required other than general support and observation. For amounts greater than 250 mg/kg, induce gastric emptying.
In five anuric patients, it was demonstrated that an average of 63% of a 1 g oral dose is extracted from the body during a 6 to 8 hour hemodialysis session.
-
DOSAGE AND ADMINISTRATION
Cefadroxil for oral suspension is acid-stable and may be administered orally without regard to meals. Administration with food may be helpful in diminishing potential gastrointestinal complaints occasionally associated with oral cephalosporin therapy.
Adults
For uncomplicated lower urinary tract infections (i.e., cystitis) the usual dosage is 1 or 2 g per day in a single (q.d.) or divided doses (b.i.d.).
For all other urinary tract infections the usual dosage is 2 g per day in divided doses (b.i.d.).
Skin and Skin Structure Infections
For skin and skin structure infections the usual dosage is 1 g per day in single (q.d.) or divided doses (b.i.d.).
Pharyngitis and Tonsillitis
Treatment of group A beta-hemolytic streptococcal pharyngitis and tonsillitis— 1 g per day in single (q.d.) or divided doses (b.i.d.) for 10 days.
Children
For urinary tract infections, the recommended daily dosage for children is 30 mg/kg/day in divided doses every 12 hours. For pharyngitis, tonsillitis, and impetigo, the recommended daily dosage for children is 30 mg/kg/day in a single dose or in equally divided doses every 12 hours. For other skin and skin structure infections, the recommended daily dosage is 30 mg/kg/day in equally divided doses every 12 hours. In the treatment of beta-hemolytic streptococcal infections, a therapeutic dosage of cefadroxil for oral suspension should be administered for at least 10 days.
See chart for total daily dosage for children.
DAILY DOSAGE OF CEFADROXIL FOR ORAL SUSPENSION
Child’s Weight
lbs
kg
250 mg/5 mL
500 mg/5 mL
10
4.5
½ tsp
-
20
9.1
1 tsp
-
30
13.6
1½ tsp
-
40
18.2
2 tsp
1 tsp
50
22.7
2½ tsp
1¼ tsp
60
27.3
3 tsp
1½ tsp
70 & above
31.8 +
--
2 tsp
Renal Impairment
In patients with renal impairment, the dosage of cefadroxil monohydrate should be adjusted according to creatinine clearance rates to prevent drug accumulation. The following schedule is suggested. In adults, the initial dose is 1000 mg of cefadroxil monohydrate and the maintenance dose (based on the creatinine clearance rate [mL/min/1.73 m2]) is 500 mg at the time intervals listed below.
Creatinine Clearances
Dosage Interval
0 to 10 mL/min
36 hours
10 to 25 mL/min
24 hours
25 to 50 mL/min
12 hours
Patients with creatinine clearance rates over 50 mL/min may be treated as if they were patients having normal renal function.
Reconstitution Directions for Oral Suspension
Bottle Size
Reconstitution Directions
100 mL
Suspend in a total of 67 mL water.
Method: Tap bottle lightly to loosen powder.
Add 67 mL of water in two portions.
Shake well after each addition.
75 mL
Suspend in a total of 51 mL water.
Method: Tap bottle lightly to loosen powder.
Add 51 mL of water in two portions.
Shake well after each addition.
50 mL
Suspend in a total of 34 mL water.
Method: Tap bottle lightly to loosen powder..
Add 34 mL of water in two portions.
Shake well after each addition
After reconstitution, store in refrigerator. Shake well before using.
Keep container tightly closed. Discard unused portion after 14 days.
-
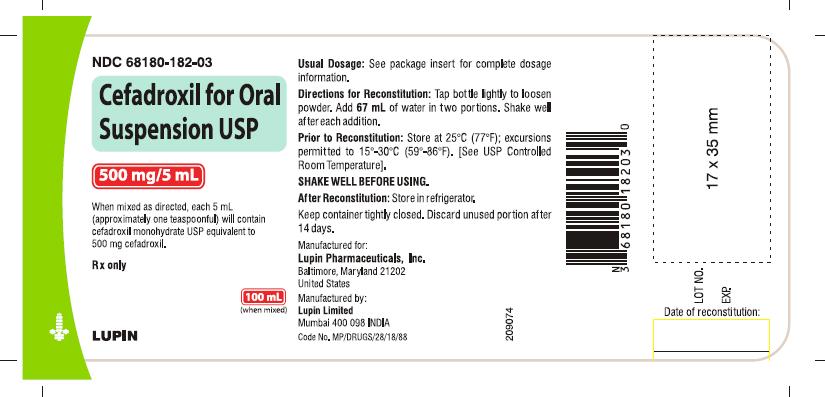
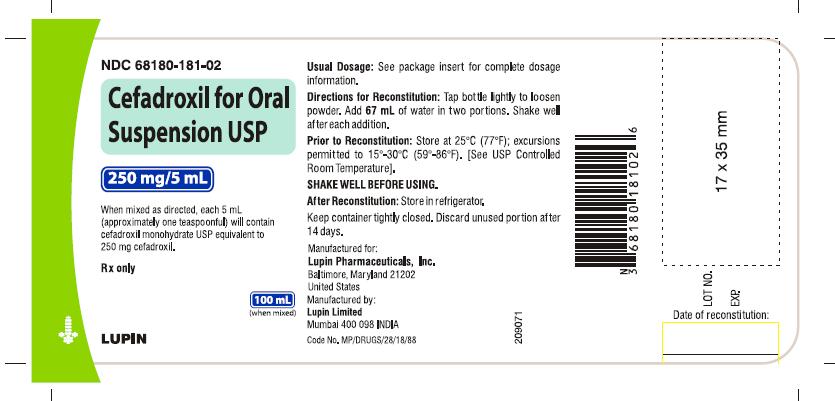
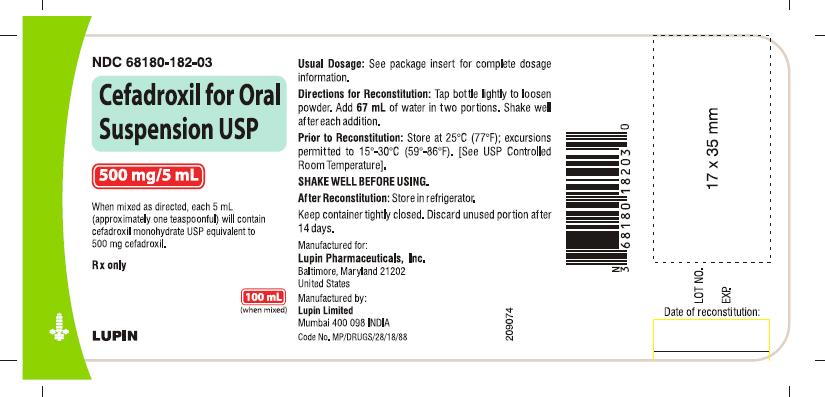
HOW SUPPLIED
Cefadroxil for oral suspension USP is an orange-pineapple flavored, and is supplied as follows:
250 mg/5 mL NDC 68180-181-01 50 mL Bottle
NDC 68180-181-02 100 mL Bottle
500 mg/5 mL NDC 68180-182-01 50 mL Bottle
NDC 68180-182-02 75 mL Bottle
NDC 68180-182-03 100 mL Bottle
Prior to reconstitution: Store at 25°C (77°F); excursions permitted to 15° to 30° C (59° to 86° F). [See USP Controlled Room Temperature].
After reconstitution: Store in refrigerator. Shake well before using. Keep container tightly closed. Discard unused portion after 14 days.
-
REFERENCES
- Clinical and Laboratory Standards Institute, Approved Standard, Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard – Eleventh Edition, Vol. 32 (1): M02- A11, Wayne, PA, January, 2012.
- Clinical and Laboratory Standards Institute, Approved Standard: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard – Ninth Edition, Vol. 32 (2): M07-A9, Wayne, PA, January, 2012.
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. CLSI Document M100-S22, Vol. 32, No. 3, CLSI, Wayne, PA, January, 2012.
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States
Manufactured by:
Lupin Limited
Mandideep 462 046
INDIA
Revised: May 2014 ID#: 237061
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CEFADROXIL
cefadroxil powder, for suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68180-181 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFADROXIL (UNII: 280111G160) (CEFADROXIL ANHYDROUS - UNII:Q525PA8JJB) CEFADROXIL ANHYDROUS 250 mg in 5 mL Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 6 (UNII: H77VEI93A8) ORANGE (UNII: 5EVU04N5QU) PINEAPPLE (UNII: 2A88ZO081O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCROSE (UNII: C151H8M554) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color ORANGE (Orange) Score Shape Size Flavor ORANGE (Orange-Pineapple) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68180-181-02 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/23/2008 2 NDC:68180-181-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/23/2008 02/28/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065396 04/23/2008 CEFADROXIL
cefadroxil powder, for suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68180-182 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFADROXIL (UNII: 280111G160) (CEFADROXIL ANHYDROUS - UNII:Q525PA8JJB) CEFADROXIL ANHYDROUS 500 mg in 5 mL Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 6 (UNII: H77VEI93A8) ORANGE (UNII: 5EVU04N5QU) PINEAPPLE (UNII: 2A88ZO081O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCROSE (UNII: C151H8M554) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color ORANGE (Orange) Score Shape Size Flavor ORANGE (Orange-Pineapple) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68180-182-02 75 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/23/2008 2 NDC:68180-182-03 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/23/2008 3 NDC:68180-182-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2040 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065396 04/23/2008 Labeler - Lupin Pharmaceuticals, Inc. (089153071) Registrant - LUPIN LIMITED (675923163) Establishment Name Address ID/FEI Business Operations LUPIN LIMITED 725504448 MANUFACTURE(68180-181, 68180-182)