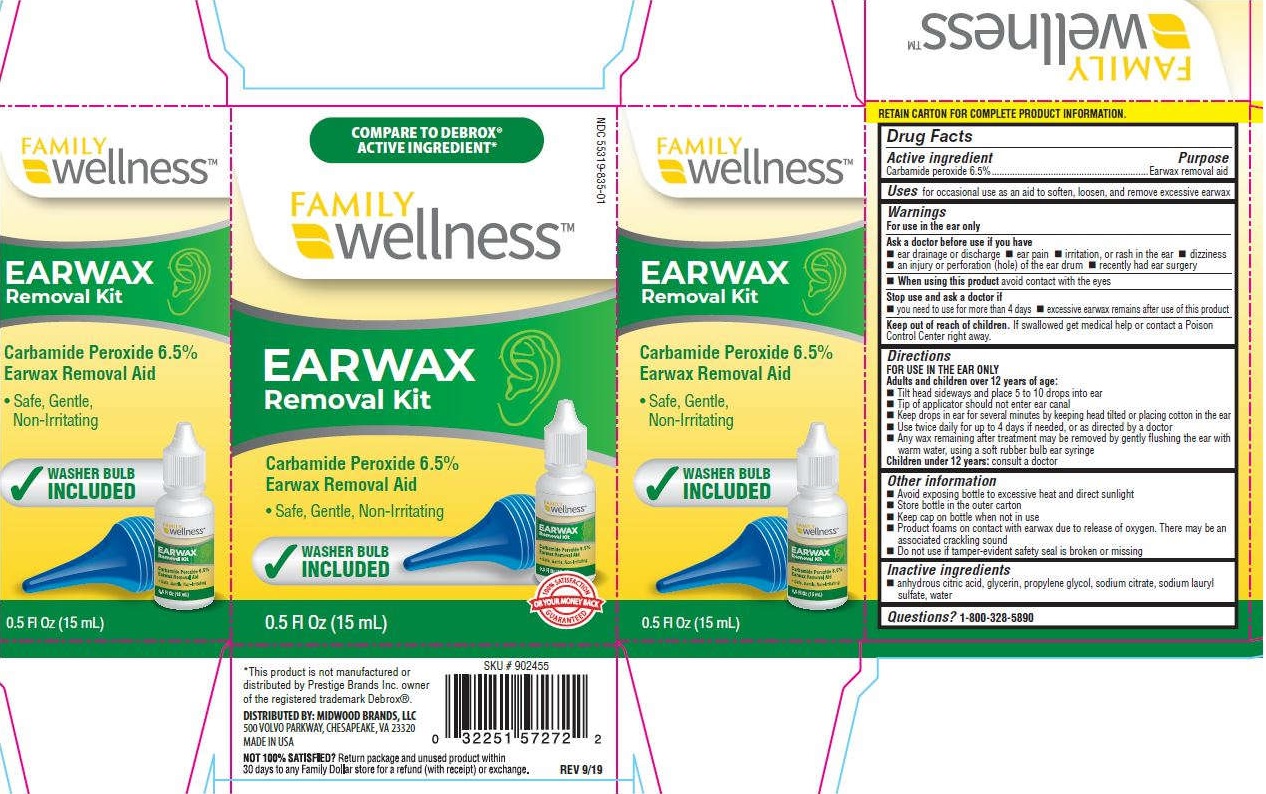
Label: FAMILY WELLNESS EARWAX REMOVAL EARWAX AND BULB- carbamide peroxide kit
- NDC Code(s): 55319-835-01, 55319-836-01
- Packager: Family Dollar Services Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
-
Warnings
For use in the ear only
Ask a doctor before use if you have
- ear drainage or discharge
- ear pain
- irritation, or rash in the ear
- dizziness
- an injury or perforation (hole) of the ear drum
- recently had ear surgery
-
Directions
FOR USE IN THE EAR ONLY.
Adults and children over 12 years of age:
- Tilt head sideways and place 5 to 10 drops into ear
- Tip of applicator should not enter ear canal
- Keep drops in ear for several minutes by keeping head tilted or placing cotton in the ear
- Use twice daily for up to 4 days if needed, or as directed by a doctor
- Any wax remaining after treatment may be removed by gently flusing the ear with warm water, using a soft rubber bulb ear syringe
Children under 12 years: consult a doctor
- Other information
- Inactive ingredients
- Questions?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
FAMILY WELLNESS EARWAX REMOVAL EARWAX AND BULB
carbamide peroxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55319-835 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55319-835-01 1 in 1 KIT 11/01/2019 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKAGE 15 mL Part 1 of 1 FAMILY WELLNESS EARWAX REMOVAL
carbamide peroxide solution/ dropsProduct Information Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBAMIDE PEROXIDE (UNII: 31PZ2VAU81) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) CARBAMIDE PEROXIDE 65 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM LAURYL SULFATE (UNII: 368GB5141J) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55319-836-01 1 in 1 BOX 1 15 mL in 1 PACKAGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M014 11/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M014 11/01/2019 Labeler - Family Dollar Services Inc (024472631) Establishment Name Address ID/FEI Business Operations Bell Pharmaceuticals, Inc. 140653770 manufacture(55319-835)