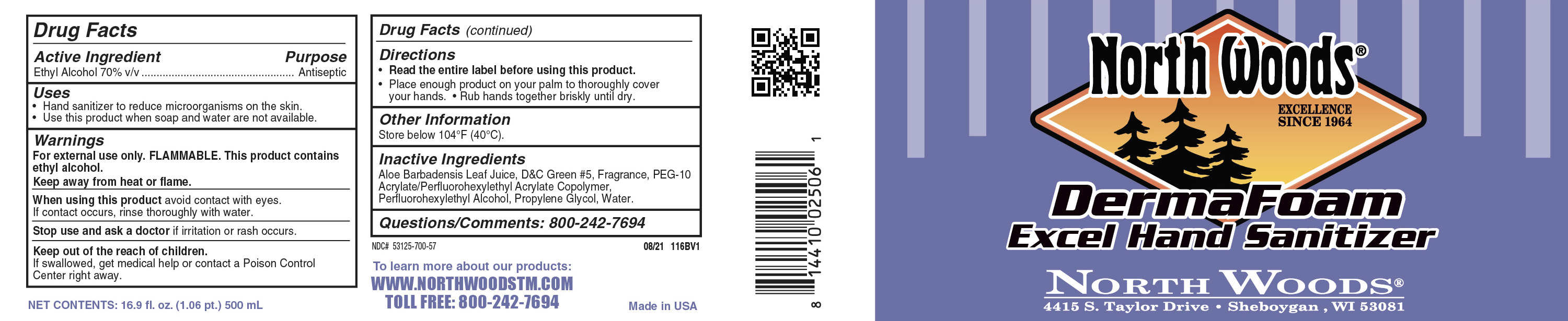
Label: ETHANOL- derma foam excel hand sanitizer soap
- NDC Code(s): 53125-803-88
- Packager: Superior Chemical Co DBA NorthWoods
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredientEthyl Alcohol 70% V/V
-
Warning statementFore external use only. FLAMMABLE. This product contains ethyl alcohol. Keep away from heat or flame
-
purposeAntiseptic
-
when using directionswhen using this product avoid contact with eyes. if contact occurs, rinsth thoroughly wiht water. Stop use and ask a doctor if irriataion or rash occurs
-
inactive ingredientsAloe Barbadensis Leaf Juide, D&C Green #5, Fragrance, PER-10 Acrylate/perfluorohexylethly Acrylate copolymer, Perfluoroheexylethyl alcohol, propylene glycol, water
-
keep out of reach of children wordingKeep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
-
Directions for usage and dosageRead entire label before using this product - Place enough product on your palm to thoroughly cover your hands. Rub hands together briskly until dry
-
indications and usageHand sanitizer to reduce microorganisms on the skin. Use this product when soap and water are not available
-
principle label
-
INGREDIENTS AND APPEARANCEProduct Information

