Label: AMOXICILLIN AND CLAVULANATE POTASSIUM tablet, film coated
- NDC Code(s): 82868-067-14
- Packager: Northwind Pharmaceuticals, LLC
- This is a repackaged label.
- Source NDC Code(s): 65862-503
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AMOXICILLIN and CLAVULANATE POTASSIUM TABLETS safely and effectively. See full prescribing information for AMOXICILLIN and ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAmoxicillin and clavulanate potassium tablets are indicated for the treatment of infections in adults and pediatric patients, due to susceptible isolates of the designated bacteria in the ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - Amoxicillin and clavulanate potassium tablets may be taken without regard to meals; however, absorption of clavulanate potassium is enhanced when ...
-
3 DOSAGE FORMS AND STRENGTHS250 mg/125 mg:White to off-white, oval shaped, film-coated tablets, debossed with ‘A’ on one side and ‘63’ on the other side, contains 250 mg of amoxicillin USP as the trihydrate and 125 mg of ...
-
4 CONTRAINDICATIONS4.1 Serious Hypersensitivity Reactions - Amoxicillin and clavulanate potassium tablets are contraindicated in patients with a history of serious hypersensitivity reactions (e.g., anaphylaxis or ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving beta-lactam antibacterials, including ...
-
6 ADVERSE REACTIONSThe following are discussed in more detail in other sections of the labeling: Anaphylactic reactions - [see - Warnings and Precautions (5.1)] Severe Cutaneous Adverse ...
-
7 DRUG INTERACTIONS7.1 Probenecid - Probenecid decreases the renal tubular secretion of amoxicillin but does not delay renal excretion of clavulanic acid. Concurrent use with amoxicillin and clavulanate potassium ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects: Pregnancy Category B. Reproduction studies performed in pregnant rats and mice given amoxicillin and clavulanate potassium (2:1 ratio formulation of ...
-
10 OVERDOSAGEIn case of overdosage, discontinue medication, treat symptomatically, and institute supportive measures as required. A prospective study of 51 pediatric patients at a poison-control center ...
-
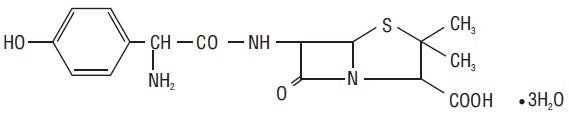
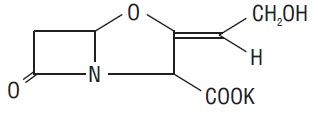
11 DESCRIPTIONAmoxicillin and clavulanate potassium tablets, USP are an oral antibacterial combination consisting of amoxicillin and the beta-lactamase inhibitor, clavulanate potassium (the potassium salt of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Amoxicillin and clavulanate potassium is an antibacterial drug - [see - Microbiology (12.4)] . 12.3 Pharmacokinetics - Mean amoxicillin and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate carcinogenic potential. Amoxicillin and clavulanate ...
-
14 CLINICAL STUDIES14.1 Lower Respiratory Tract and Complicated Urinary Tract Infections - Data from 2 pivotal trials in 1,191 patients treated for either lower respiratory tract infections or complicated urinary ...
-
15 REFERENCES1. Swanson-Biearman B, Dean BS, Lopez G, Krenzelok EP. The effects of penicillin and cephalosporin ingestions in children less than six years of age. Vet Hum Toxicol. 1988; 30: 66-67.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAmoxicillin and Clavulanate Potassium Tablets USP, 875 mg/125 mg are white to off-white, capsule shaped, film-coated tablets, debossed with ‘X’ on one side and score line in between 3 and 2 on ...
-
17 PATIENT COUNSELING INFORMATIONAdministration Instructions - Inform patients that amoxicillin and clavulanate potassium tablets may be taken every 8 hours or every 12 hours, depending on the dose prescribed. Each dose should be ...
-
Principal Display PanelNDC: 82868-067-14
-
INGREDIENTS AND APPEARANCEProduct Information