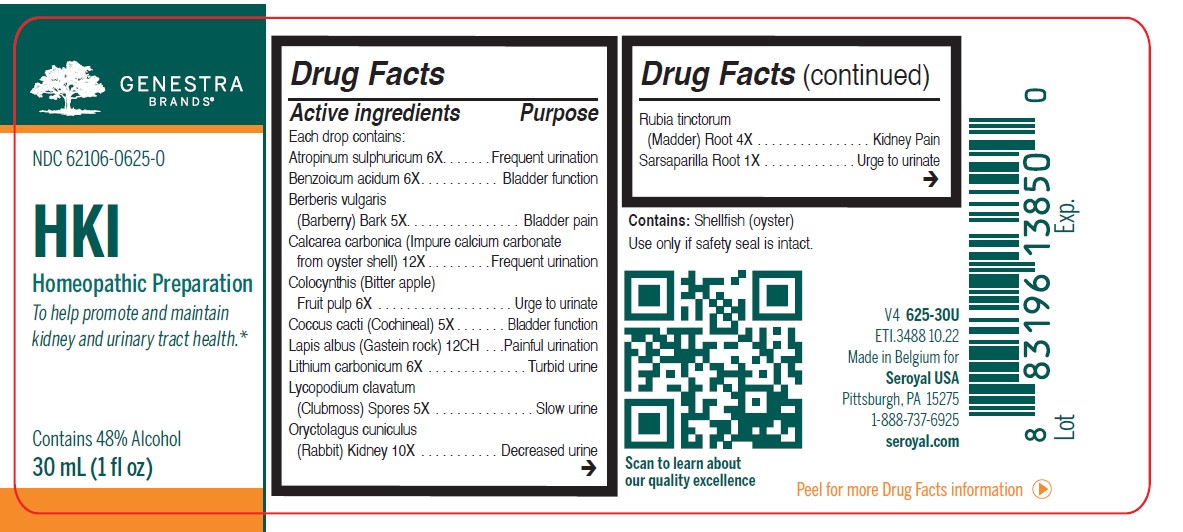
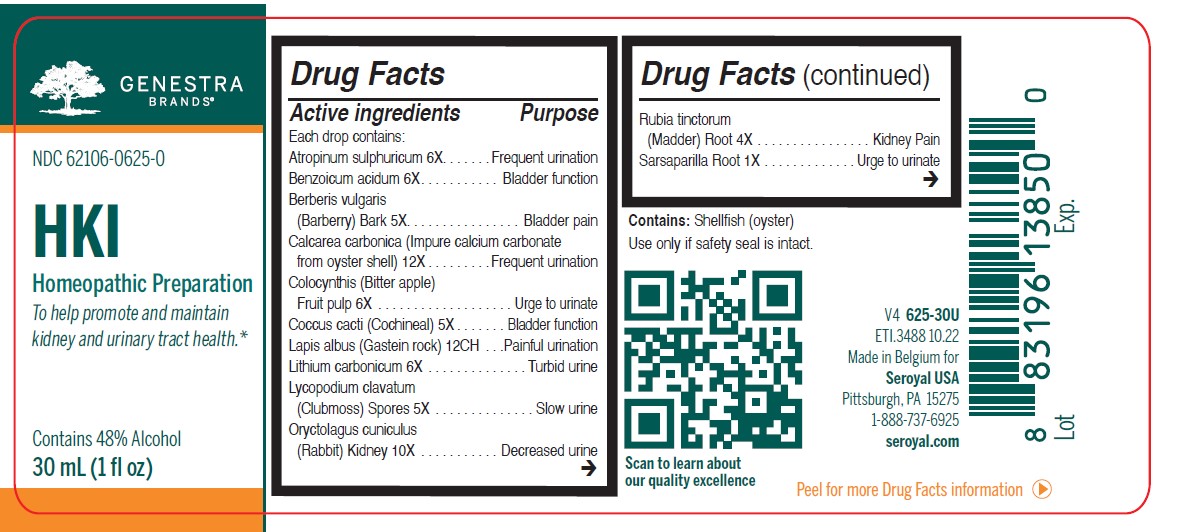
Label: HKI- atropinum sulphuricum, benzoicum acidum, berberis vulgaris, calcarea carbonica, colocynthis, coccus cacti, lapis albus, lithium carbonicum, lycopodium clavatum, oryctolagus cuniculus kidney, rubia tinctorum, sarsaparilla, urtica urens liquid
- NDC Code(s): 62106-0625-0
- Packager: Seroyal USA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Atropinum sulphuricum 6X
Benzoicum acidum 6X
Berberis vulgaris (Barberry) Bark 5X
Calcarea carbonica 12X
Colocynthis (Bitter apple) Dried fruit 6XCoccus cacti (Cochineal) 5X
Lapis albus (Gastein rock) 12CH
Lithium carbonicum 6X
Lycopodium clavatum (Clubmoss) Spores 5X
Oryctolagus cuniculus (Rabbit) Kidney 10XRubia tinctorum (Madder) Root 4X
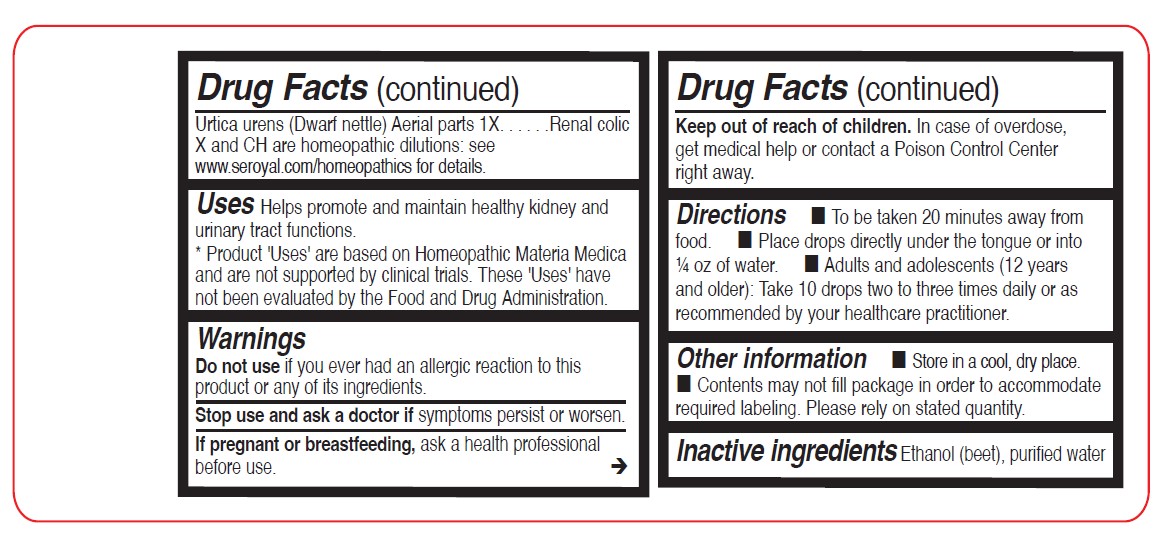
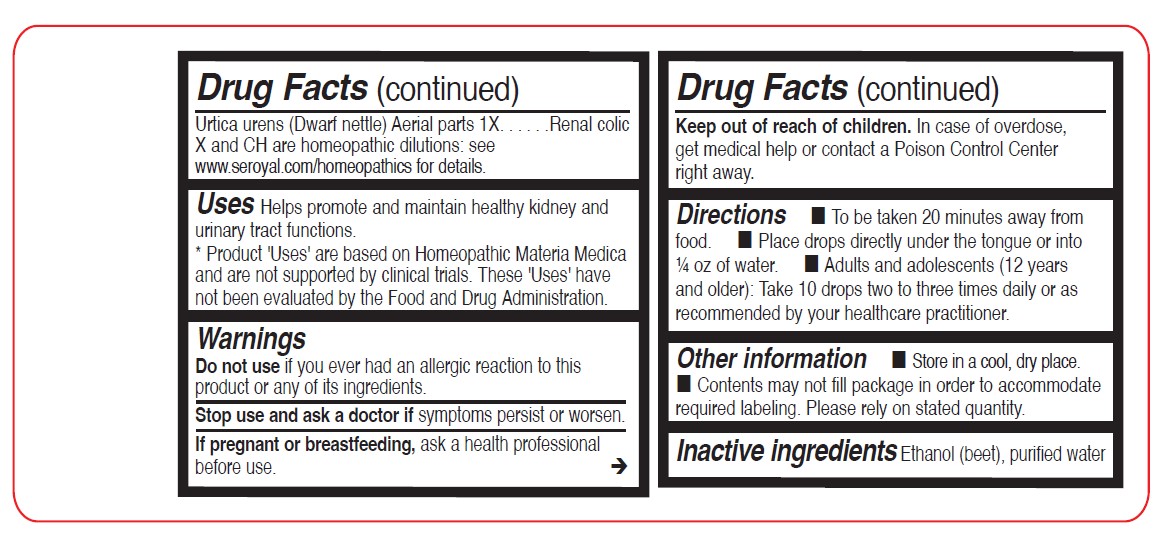
Sarsaparilla Root 1XUrtica urens (Dwarf Nettle) Aerial parts 1X
- PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DO NOT USE
-
DOSAGE & ADMINISTRATION
Directions
To be taken 20 minutes away from food.
Place drops directly under the tongue or into ¼ oz of water.
Adults and adolescents (12 years and older): Take 10 drops two to three times daily or as recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your healthcare practitioner.
-
INDICATIONS & USAGE
Uses
Helps promote and maintain healthy kidney and urinary tract functions.
Directions
To be taken 20 minutes away from food.
Place drops directly under the tongue or into ¼ oz of water.
Adults and adolescents (12 years and older): Take 10 drops two to three times daily or as recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your healthcare practitioner.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HKI
atropinum sulphuricum, benzoicum acidum, berberis vulgaris, calcarea carbonica, colocynthis, coccus cacti, lapis albus, lithium carbonicum, lycopodium clavatum, oryctolagus cuniculus kidney, rubia tinctorum, sarsaparilla, urtica urens liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-0625 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPINE SULFATE (UNII: 03J5ZE7KA5) (ATROPINE - UNII:7C0697DR9I) ATROPINE SULFATE 6 [hp_X] in 30 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 12 [hp_X] in 30 mL CITRULLUS COLOCYNTHIS FRUIT PULP (UNII: 23H32AOH17) (CITRULLUS COLOCYNTHIS FRUIT PULP - UNII:23H32AOH17) CITRULLUS COLOCYNTHIS FRUIT PULP 6 [hp_X] in 30 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 5 [hp_X] in 30 mL ORYCTOLAGUS CUNICULUS KIDNEY (UNII: 482QQ7H44Q) (ORYCTOLAGUS CUNICULUS KIDNEY - UNII:482QQ7H44Q) ORYCTOLAGUS CUNICULUS KIDNEY 10 [hp_X] in 30 mL SARSAPARILLA (UNII: 2H1576D5WG) (SARSAPARILLA - UNII:2H1576D5WG) SARSAPARILLA 1 [hp_X] in 30 mL CALCIUM HEXAFLUOROSILICATE (UNII: 2NVP93XVQ3) (CALCIUM HEXAFLUOROSILICATE - UNII:2NVP93XVQ3) CALCIUM HEXAFLUOROSILICATE 12 [hp_C] in 30 mL BENZOIC ACID (UNII: 8SKN0B0MIM) (BENZOIC ACID - UNII:8SKN0B0MIM) BENZOIC ACID 6 [hp_X] in 30 mL PROTORTONIA CACTI (UNII: LZB7TFX1LT) (PROTORTONIA CACTI - UNII:LZB7TFX1LT) PROTORTONIA CACTI 5 [hp_X] in 30 mL LITHIUM CARBONATE (UNII: 2BMD2GNA4V) (LITHIUM CATION - UNII:8H8Z5UER66) LITHIUM CARBONATE 6 [hp_X] in 30 mL RUBIA TINCTORUM ROOT (UNII: 0SVP95L23G) (RUBIA TINCTORUM ROOT - UNII:0SVP95L23G) RUBIA TINCTORUM ROOT 4 [hp_X] in 30 mL URTICA URENS (UNII: IHN2NQ5OF9) (URTICA URENS - UNII:IHN2NQ5OF9) URTICA URENS 1 [hp_X] in 30 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 5 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-0625-0 1 in 1 CARTON 12/14/2015 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/14/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN'UP 401010287 manufacture(62106-0625)