Label: DERMAWERX SURGICAL PLUS PAK- mupirocin ointment kit kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 0116-1061-08, 45802-112-22, 69329-253-02, 69329-270-01 - Packager: Patchwerx Labs, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 9, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Mupirocin Ointment USP, 2%
For Dermatologic Use
Rx Only
DESCRIPTION
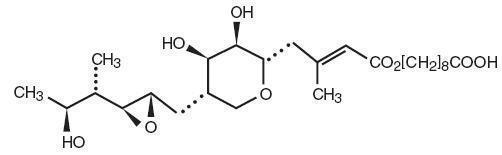
Each gram of Mupirocin Ointment USP, 2% contains 20 mg mupirocin in a bland water miscible ointment base (polyethylene glycol ointment, NF) consisting of polyethylene glycol 400 and polyethylene glycol 3350. Mupirocin is a naturally occurring antibiotic. The chemical name is ( E)-(2 S,3 R,4 R,5 S)-5-[(2 S,3 S,4 S,5 S)-2,3-Epoxy-5-hydroxy-4-methylhexyl]tetrahydro-3,4-dihydroxy-β-methyl-2H-pyran-2-crotonic acid, ester with 9-hydroxynonanoic acid. The molecular formula of mupirocin is C 26H 44O 9 and the molecular weight is 500.62. The chemical structure is:
CLINICAL PHARMACOLOGY
Application of 14C-labeled mupirocin ointment to the lower arm of normal male subjects followed by occlusion for 24 hours showed no measurable systemic absorption (<1.1 nanogram mupirocin per milliliter of whole blood). Measurable radioactivity was present in the stratum corneum of these subjects 72 hours after application.
Following intravenous or oral administration, mupirocin is rapidly metabolized. The principal metabolite, monic acid, is eliminated by renal excretion, and demonstrates no antibacterial activity. In a study conducted in 7 healthy adult male subjects, the elimination half-life after intravenous administration of mupirocin was 20 to 40 minutes for mupirocin and 30 to 80 minutes for monic acid. The pharmacokinetics of mupirocin has not been studied in individuals with renal insufficiency.
Microbiology -
Mupirocin is an antibacterial agent produced by fermentation using the organism Pseudomonas fluorescens. It is active against a wide range of gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA). It is also active against certain gram-negative bacteria. Mupirocin inhibits bacterial protein synthesis by reversibly and specifically binding to bacterial isoleucyl transfer-RNA synthetase. Due to this unique mode of action, mupirocin demonstrates no in vitro cross-resistance with other classes of antimicrobial agents.Resistance occurs rarely. However, when mupirocin resistance does occur, it appears to result from the production of a modified isoleucyl-tRNA synthetase. High-level plasmid-mediated resistance (MIC > 1024 mcg/mL) has been reported in some strains of S. aureus and coagulase-negative staphylococci.
Mupirocin is bactericidal at concentrations achieved by topical administration. However, the minimum bactericidal concentration (MBC) against relevant pathogens is generally 8-fold to 30-fold higher than the minimum inhibitory concentration (MIC). In addition, mupirocin is highly protein bound (>97%), and the effect of wound secretions on the MICs of mupirocin has not been determined.
Mupirocin has been shown to be active against most strains of S. aureus and Streptococcus pyogenes, both in vitro and in clinical studies (see INDICATIONS AND USAGE). The following in vitro data are available, BUT THEIR CLINICAL SIGNIFICANCE IS UNKNOWN. Mupirocin is active against most strains of Staphylococcus epidermidis and Staphylococcus saprophyticus.
INDICATIONS AND USAGE
Mupirocin Ointment USP, 2% is indicated for the topical treatment of impetigo due to: S. aureus and S. pyogenes.
CONTRAINDICATIONS
This drug is contraindicated in individuals with a history of sensitivity reactions to any of its components.
PRECAUTIONS
If a reaction suggesting sensitivity or chemical irritation should occur with the use of Mupirocin Ointment USP, 2%, treatment should be discontinued and appropriate alternative therapy for the infection instituted.
As with other antibacterial products, prolonged use may result in overgrowth of nonsusceptible organisms, including fungi.
Mupirocin Ointment USP, 2% is not formulated for use on mucosal surfaces. Intranasal use has been associated with isolated reports of stinging and drying. A paraffin-based formulation - *Bactroban Nasal ® (mupirocin calcium ointment) – is available for intranasal use.
Polyethylene glycol can be absorbed from open wounds and damaged skin and is excreted by the kidneys. In common with other polyethylene glycol-based ointments, Mupirocin Ointment USP, 2% should not be used in conditions where absorption of large quantities of polyethylene glycol is possible, especially if there is evidence of moderate or severe renal impairment.
Information for Patients -
Use this medication only as directed by your healthcare provider. It is for external use only. Avoid contact with the eyes. The medication should be stopped and your healthcare practitioner contacted if irritation, severe itching, or rash occurs. If impetigo has not improved in 3 to 5 days, contact your healthcare practitioner.Drug Interactions -
The effect of the concurrent application of Mupirocin Ointment USP, 2% and other drug products has not been studied.Carcinogenesis, Mutagenesis, Impairment of Fertility -
Long-term studies in animals to evaluate carcinogenic potential of mupirocin have not been conducted.Results of the following studies performed with mupirocin calcium or mupirocin sodium in vitro and in vivo did not indicate a potential for genotoxicity: Rat primary hepatocyte unscheduled DNA synthesis, sediment analysis for DNA strand breaks, Salmonella reversion test (Ames), Escherichia coli mutation assay, metaphase analysis of human lymphocytes, mouse lymphoma assay, and bone marrow micronuclei assay in mice.
Reproduction studies were performed in male and female rats with mupirocin administered subcutaneously at doses up to 14 times a human topical dose (approximately 60 mg mupirocin per day) on a mg/m 2 basis and revealed no evidence of impaired fertility and reproductive performance from mupirocin.
Pregnancy:
Teratogenic Effects -
Reproduction studies have been performed in rats and rabbits with mupirocin administered subcutaneously at doses up to 22 and 43 times, respectively, the human topical dose (approximately 60 mg mupirocin per day) on a mg/m 2 basis and revealed no evidence of harm to the fetus due to mupirocin. There are however, no adequate and well-controlled studies in pregnant women. Because animal studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.Nursing Mothers -
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Mupirocin Ointment USP, 2% is administered to a nursing woman.Pediatric Use -
The safety and effectiveness of Mupirocin Ointment USP, 2% have been established in the age range of 2 months to 16 years. Use of mupirocin ointment USP, 2% in these age groups is supported by evidence from adequate and well-controlled studies of mupirocin ointment USP, 2% in impetigo in pediatric patients studied as part of the pivotal clinical trials (see CLINICAL STUDIES).ADVERSE REACTIONS
The following local adverse reactions have been reported in connection with the use of mupirocin ointment USP, 2%: Burning, stinging, or pain in 1.5% of patients; itching in 1% of patients; rash, nausea, erythema, dry skin, tenderness, swelling, contact dermatitis, and increased exudate in less than 1% of patients. Systemic reactions to mupirocin ointment USP, 2% have occurred rarely.
DOSAGE AND ADMINISTRATION
A small amount of Mupirocin Ointment USP, 2% should be applied to the affected area 3 times daily. The area treated may be covered with a gauze dressing if desired. Patients not showing a clinical response within 3 to 5 days should be re-evaluated.
CLINICAL STUDIES
The efficacy of topical mupirocin ointment USP, 2% in impetigo was tested in two studies. In the first, patients with impetigo were randomized to receive either mupirocin ointment USP, 2% or vehicle placebo three times daily for 8 to 12 days. Clinical efficacy rates at end of therapy in the evaluable populations (adults and pediatric patients included) were 71% for mupirocin ointment USP, 2% (n=49) and 35% for vehicle placebo (n=51). Pathogen eradication rates in the evaluable populations were 94% for mupirocin ointment USP, 2% and 62% for vehicle placebo. There were no side effects reported in the group receiving mupirocin ointment USP, 2%.
In the second study, patients with impetigo were randomized to receive either mupirocin ointment USP, 2% three times daily or 30 to 40 mg/kg oral erythromycin ethylsuccinate per day (this was an unblinded study) for 8 days. There was a follow-up visit 1 week after treatment ended. Clinical efficacy rates at the follow-up visit in the evaluable populations (adults and pediatric patients included) were 93% for mupirocin ointment USP, 2% (n=29) and 78.5% for erythromycin (n=28). Pathogen eradication rates in the evaluable patient populations were 100% for both test groups. There were no side effects reported in the group receiving mupirocin ointment.
Pediatrics -
There were 91 pediatric patients aged 2 months to 15 years in the first study described above. Clinical efficacy rates at end of therapy in the evaluable populations were 78% for mupirocin ointment USP, 2% (n=42) and 36% for vehicle placebo (n=49). In the second study described above, all patients were pediatric except two adults in the group receiving mupirocin ointment USP, 2%. The age range of the pediatric patients was 7 months to 13 years. The clinical efficacy rate for mupirocin ointment USP, 2% (n=27) was 96%, and for erythromycin it was unchanged (78.5%). -
Chlorhexidine Gluconate Solution, 4%
Drug Facts
Uses
- surgical hand scrub: significantly reduces the number of microorganisms on the hands and forearms prior to surgery or patient care
- healthcare personnel handwash: helps reduce bacteria that potentially can cause disease
- patient preoperative skin preparation: for the preparation of the patient's skin prior to surgeryskin wound and general skin cleansing
- skin wound and general skin cleansing
Warnings
For external use only.
Do not use
- if you are allergic to chlorhexidine gluconate or any other ingredients
- in contact with meninges
- in the genital area
- as a preoperative skin preparation of the head or face
When using this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if placed or kept in the eye during surgical procedures or may cause deafness when instilled in the middle ear through perforated eardrums.
- if solution should contact these areas, rinse out promptly and thoroughly with water
- wounds which involve more than the superficial layers of the skin should not be routinely treated
- repeated general skin cleansing of large body areas should not be done except when the underlying condition makes it necessary to reduce the bacterial population of skin
Stop use and ask a doctor
if irritation, sensitization or allergic reaction occurs. These may be signs of a serious condition.Keep out of reach of children
if swallowed, get medical help or contact a Poison Control Center right away.Directions
- use with care in premature infants and infants under 2 months of age. These products may cause irritation or chemical burns.
Surgical hand scrub:
- wet hands and forearms with water
- scrub for 3 minutes with about 5 ml of product and a wet brush paying close attention to the nails, cuticles and interdigital spaces
- a separate nail cleaner may be used
- rinse thoroughly
- wash for an additional 3 minutes with 5 ml of product and rinse under running water
- dry thoroughly
Healthcare personnel handwash:
- wet hands with water
- dispense about 5 ml of product into cupped hands and wash in a vigorous manner for 15 seconds
- rinse and dry thoroughly
Patient preoperative skin preparation:
- apply product liberally to surgical site and swab for at least 2 minutes and dry with a sterile towel
- repeat procedure for an additional 2 minutes and dry with a sterile towel
Skin wound and general skin cleaning:
- thoroughly rinse the area to be cleaned with water
- apply the minimum amount of product necessary to cover the skin or wound area and wash gently
- rinse again thoroughly
-
Skin Repair Cream (5% Dimethicone)
Drug Facts
Warnings
For external use only
Do not use on
- deep or puncture wounds
- animal bites
- serious burns
When using this product
- do not get into eyes
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
- If swallowed, get medical help or contact a Poison Control Center right away.
Inactive ingredients
Aleurites moluccana seed oil, Aloe barbadensis ( Aloe vera) leaf juice, butylene glycol, caprylyl glycol, Carthamus tinctorius (safflower) seed oil, cetyl alcohol, chlorphenesin, dimethicone crosspolymer, disodium EDTA, fragrance, glycerin, glyceryl stearate, Complex [consisting of: bisabolol, calcium pantothenate (vitamin B5), Carthamus tinctorius (safflower) oleosomes, maltodextrin, niacinamide (vitamin B3), pyridoxine HCl (vitamin B6 ), silica, sodium ascorbyl phosphate (vitamin C), sodium starch octenylsuccinate, tocopheryl acetate (vitamin E), Zingiber officinale (ginger) root extract], PEG-100 stearate, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, phenoxyethanol, purified water, sodium hyaluronate, stearic acid, triethanolamine
-
Silicone Tape
Uses
• To be applied to wounds or scars as a protective silicone barrier.
• As a dressing for abrasions, surgical wounds, donor sites, lacerations, ulcers, skin tears, superficial partial thickness burns, venous leg ulcers.
• As a dressing/securement for IV related uses, pressure ulcers, skin care, and wound carePrecautions
• Do not use if you are allergic to silicone
• Keep out of reach of childrenDirections for use
• Apply tape to wound or scar as needed or as directed by your physician. Remove tape, wash area, and apply new tape at least every 24 hours. - Dermawerx Surgical Plus Pak
-
INGREDIENTS AND APPEARANCE
DERMAWERX SURGICAL PLUS PAK
mupirocin ointment kit kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69329-270 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69329-270-01 1 in 1 KIT; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 22 g Part 2 1 BOTTLE, PLASTIC 237 mL Part 3 1 TUBE 118 mL Part 1 of 3 MUPIROCIN
mupirocin ointmentProduct Information Item Code (Source) NDC:45802-112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MUPIROCIN (UNII: D0GX863OA5) (MUPIROCIN - UNII:D0GX863OA5) MUPIROCIN 20 mg in 1 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45802-112-22 22 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065123 Part 2 of 3 ANTISEPTIC SKIN CLEANSER
chlorhexidine gluconate solutionProduct Information Item Code (Source) NDC:0116-1061 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 4 g in 100 mL Inactive Ingredients Ingredient Name Strength COCO DIETHANOLAMIDE (UNII: 92005F972D) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) ISOPROPYL ALCOHOL (UNII: ND2M416302) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) WATER (UNII: 059QF0KO0R) TRIDECYL ALCOHOL (UNII: 8I9428H868) GLUCONOLACTONE (UNII: WQ29KQ9POT) PEG-75 LANOLIN (UNII: 09179OX7TB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0116-1061-08 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019125 Part 3 of 3 SKIN REPAIR COMPLEX
dimethicone creamProduct Information Item Code (Source) NDC:69329-253 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SAFFLOWER OIL (UNII: 65UEH262IS) CETYL ALCOHOL (UNII: 936JST6JCN) KUKUI NUT OIL (UNII: TP11QR7B8R) ALOE VERA LEAF (UNII: ZY81Z83H0X) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE/DIENE DIMETHICONE CROSSPOLYMER (UNII: RSA9I561OK) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LEVOMENOL (UNII: 24WE03BX2T) CALCIUM PANTOTHENATE (UNII: 568ET80C3D) CARTHAMUS TINCTORIUS (SAFFLOWER) OLEOSOMES (UNII: 9S60Q72309) MALTODEXTRIN (UNII: 7CVR7L4A2D) PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) TROLAMINE (UNII: 9O3K93S3TK) STEARIC ACID (UNII: 4ELV7Z65AP) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PHENOXYETHANOL (UNII: HIE492ZZ3T) NIACINAMIDE (UNII: 25X51I8RD4) GINGER (UNII: C5529G5JPQ) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) PEG-100 STEARATE (UNII: YD01N1999R) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) CAPRYLYL GLYCOL (UNII: 00YIU5438U) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69329-253-02 118 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065123 10/21/2015 Labeler - Patchwerx Labs, Inc. (079584480)


