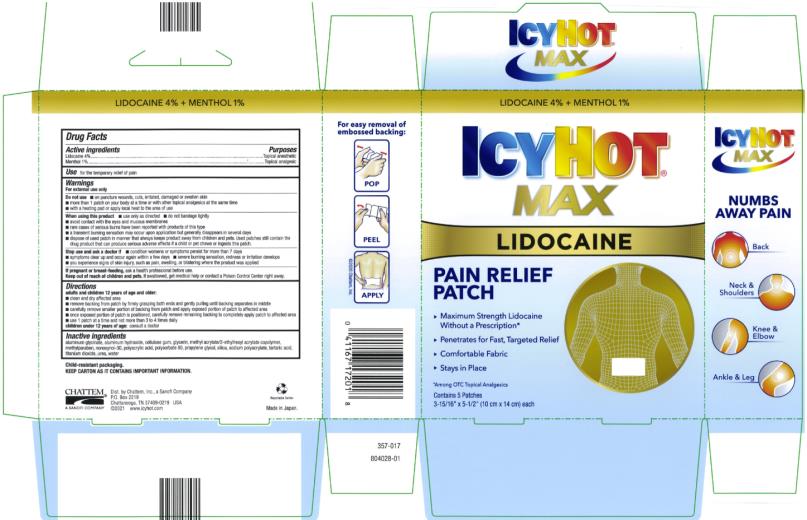
Label: ICY HOT WITH LIDOCAINE- lidocaine and menthol patch
- NDC Code(s): 41167-1720-1
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purposes
- Use
-
Warnings
For external use only
Do not use
■ on puncture wounds, cuts, irritated, damaged or swollen skin
■ more than 1 patch on your body at a time or with other topical analgesics at the same time
■ with a heating pad or apply local heat to the area of use
When using this product
■ use only as directed
■ do not bandage tightly
■ avoid contact with the eyes and mucous membranes
■ rare cases of serious burns have been reported with products of this type
■ a transient burning sensation may occur upon application but generally disappears in several days
■ dispose of used patch in manner that always keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
Stop use and ask a doctor if
■ condition worsens or symptoms persist for more than 7 days
■ symptoms clear up and occur again within a few days
■ severe burning sensation, redness or irritation develops
■ you experience signs of skin injury, such as pain, swelling, or blistering where the product was applied
-
Directions
adults and children 12 years of age and older:
■ clean and dry affected area
■ remove backing from patch by firmly grasping both ends and gently pulling until backing separates in middle
■ carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
■ once exposed portion of patch is positioned, carefully remove remaining backing to completely apply patch to affected area
■ use 1 patch at a time and not more than 3 to 4 times daily
children under 12 years of age: consult a doctor
-
Inactive ingredients
aluminum glycinate, aluminum hydroxide, cellulose gum, glycerin, methyl acrylate/2-ethylhexyl acrylate copolymer, methylparaben, nonoxynol-30, polyacrylic acid, polysorbate 80, propylene glycol, silica, sodium polyacrylate, tartaric acid, titanium dioxide, urea, water
Child-resistant packaging.
Keep carton as it contains important information.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ICY HOT WITH LIDOCAINE
lidocaine and menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-1720 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 240 mg MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 60 mg Inactive Ingredients Ingredient Name Strength 2-ETHYLHEXYL ACRYLATE (UNII: HR49R9S6XG) METHYL ACRYLATE (UNII: WC487PR91H) DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) NONOXYNOL-30 (UNII: JJX07DG188) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) UREA (UNII: 8W8T17847W) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-1720-1 5 in 1 CARTON; Type 0: Not a Combination Product 02/18/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/18/2018 Labeler - Chattem, Inc. (003336013)