Label: THERMAPATCH (TM) LIDOCAINE PAIN RELIEF PATCH- lidocaine pain relief patch patch
- NDC Code(s): 83559-006-01
- Packager: Henan Enokon Medical Instrument Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
-
Directions
Adults and children 12 years of age and over:
Clean and dry affected area
Remove film from patch and apply to the skin
Apply 1 patch at a time to affected area, not more than 3 to 4 times daily
Remove patch from the skin after at most 8 hours of application
Children under 12 years of age: consult a doctor - Other information
- Inactive ingredients
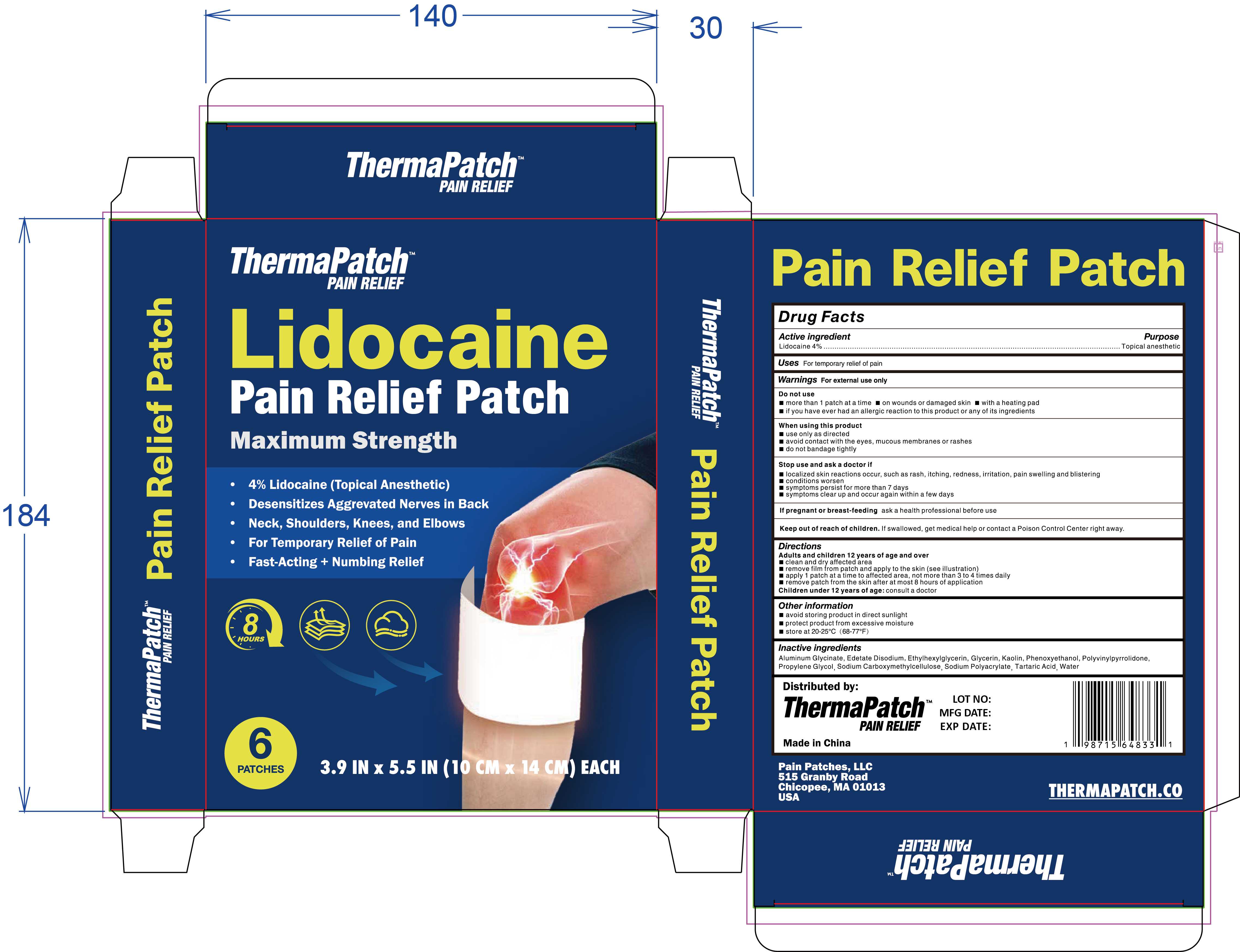
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THERMAPATCH (TM) LIDOCAINE PAIN RELIEF PATCH
lidocaine pain relief patch patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83559-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 Inactive Ingredients Ingredient Name Strength ALUMINUM GLYCINATE (UNII: 1K713C615K) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) TARTARIC ACID (UNII: W4888I119H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) KAOLIN (UNII: 24H4NWX5CO) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM ACRYLATE (UNII: 7C98FKB43H) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) EDETATE DISODIUM (UNII: 7FLD91C86K) POVIDONE (UNII: FZ989GH94E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83559-006-01 6 in 1 BOX 11/09/2024 1 1 in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/09/2024 Labeler - Henan Enokon Medical Instrument Co., Ltd. (701730676) Establishment Name Address ID/FEI Business Operations Henan Enokon Medical Instrument Co., Ltd. 701730676 manufacture(83559-006)