Label: TERRAMYCIN- oxytetracycline hydrochloride and polymyxin b sulfate ointment
- NDC Code(s): 54771-1096-1
- Packager: Zoetis Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated October 5, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
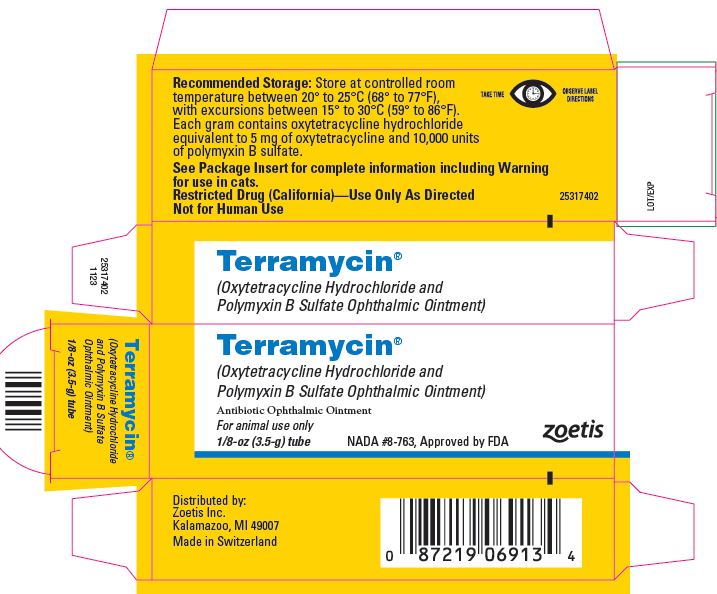
DESCRIPTION
Terramycin (Oxytetracycline Hydrochloride and
Polymyxin B Sulfate Ophthalmic Ointment) is an antibiotic,
bright yellow in color, possessing potent antimicrobial
activity. It is one of the most versatile of the broad-spectrum
antibiotics, and is effective in the treatment of infections due
to gram-positive and gram-negative bacteria, both aerobic and
anaerobic, spirochetes, rickettsiae, and certain of the larger
viruses.
Polymyxin B sulfate is one of a group of related antibiotics
derived from Bacillus polymyxa. The polymyxins are rapidly
bactericidal, this action being exclusively against
gram-negative bacteria.
The broad-spectrum effectiveness of Terramycin against both
gram-positive and gram-negative organisms is enhanced by
the particular effectiveness of polymyxin B against infections
associated with gram-negative organisms, especially those
due to Pseudomonas aeruginosa, where polymyxin B is the
antibiotic of choice. In addition, there is evidence to indicate
that polymyxin B sulfate possesses some antifungal activity.
The combined antibacterial effect of Terramycin plus polymyxin
is at least additive and, in many instances, an actual synergistic
action is obtained.
Terramycin is an ointment of oxytetracycline hydrochloride and
polymyxin B sulfate in a special petrolatum base. Each gram
of ointment contains oxytetracycline HCl equivalent to 5 mg of
oxytetracycline and 10,000 units of polymyxin B as the sulfate. -
INDICATIONS AND USAGE
Terramycin is indicated for the prophylaxis and local treatment of superficial ocular infections
due to oxytetracycline and polymyxin-sensitive organisms,
including infections due to streptococci, rickettsiae, E. coli, and
A. aerogenes, such as conjunctivitis, keratitis, pink eye, corneal
ulcer, blepharitis in dogs, cats, cattle, sheep, and horses;
ocular infections due to secondary bacterial complications of
distemper in dogs, and bacterial inflammatory conditions which
may occur secondary to other infectious diseases in the above species. -
WARNING
Serious, life threatening, hypersensitivity
(anaphylactic) reactions have been reported in some cats
following application of antibiotic ophthalmic preparations.
If your cat has swelling of the face, itching, appears weak,
vomits, or has difficulty breathing within 4 hours of application
you should discontinue treatment and contact your veterinarian
immediately.
Note: The use of oxytetracycline and other antibiotics may result
in an overgrowth of resistant organisms such as Monilia,
staphylococci, and other species of bacteria. If new infections
due to nonsensitive bacteria or fungi appear during therapy,
appropriate measures should be taken.
- DOSAGE AND ADMINISTRATION
- RECOMMENDED STORAGE
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 3.5 g Tube Label
-
INGREDIENTS AND APPEARANCE
TERRAMYCIN
oxytetracycline hydrochloride and polymyxin b sulfate ointmentProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:54771-1096 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYTETRACYCLINE HYDROCHLORIDE (UNII: 4U7K4N52ZM) (OXYTETRACYCLINE ANHYDROUS - UNII:SLF0D9077S) OXYTETRACYCLINE HYDROCHLORIDE 5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [USP'U] in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-1096-1 3.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA008763 01/02/1953 Labeler - Zoetis Inc. (828851555)