Label: ARIELLA HEMORRHOID- lidocaine, phenylephrine hcl ointment
- NDC Code(s): 83364-012-01, 83364-012-02
- Packager: YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD
- This is a repackaged label.
- Source NDC Code(s): 84010-001
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
Drug Facts
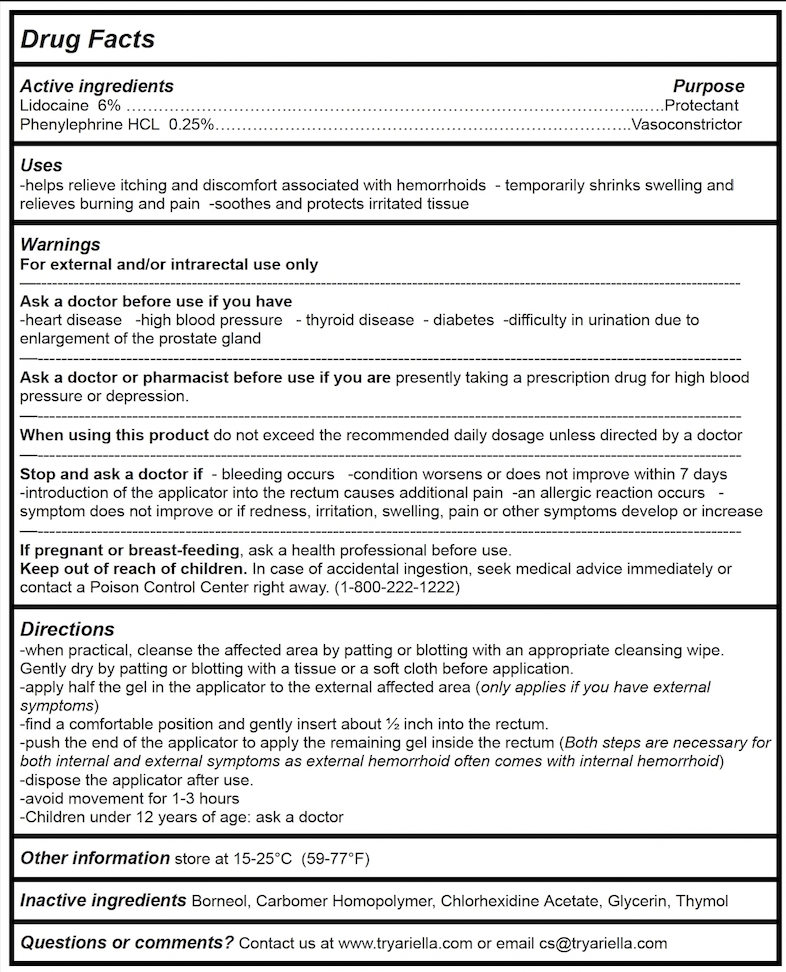
Active ingredients Purpose
Lidocaine 6% ………………………….………………………………………………………...….Protectant
Phenylephrine HCL 0.25%…………………………………………….……………………..Vasoconstrictor
Lidocaine 6% ………………………….………………………………………………………...….Protectant
Phenylephrine HCL 0.25%…………………………………………….……………………..Vasoconstrictor
Uses
-helps relieve itching and discomfort associated with hemorrhoids - temporarily shrinks swelling and relieves burning and pain -soothes and protects irritated tissue
Ask a doctor before use if you have
-heart disease -high blood pressure - thyroid disease - diabetes -difficulty in urination due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are presently taking a prescription drug for high blood pressure or depression.
Stop and ask a doctor if - bleeding occurs -condition worsens or does not improve within 7 days -introduction of the applicator into the rectum causes additional pain -an allergic reaction occurs - symptom does not improve or if redness, irritation, swelling, pain or other symptoms develop or increase
Directions
-when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a tissue or a soft cloth before application.
-apply half the gel in the applicator to the external affected area (only applies if you have external symptoms)
-find a comfortable position and gently insert about ½ inch into the rectum.
-push the end of the applicator to apply the remaining gel inside the rectum (Both steps are necessary for both internal and external symptoms as external hemorrhoid often comes with internal hemorrhoid)
-dispose the applicator after use.
-avoid movement for 1-3 hours
-Children under 12 years of age: ask a doctor
-
INGREDIENTS AND APPEARANCE
ARIELLA HEMORRHOID
lidocaine, phenylephrine hcl ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83364-012(NDC:84010-001) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 0.25 g in 100 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength BORNEOL (UNII: M89NIB437X) CHLORHEXIDINE ACETATE (UNII: 5908ZUF22Y) GLYCERIN (UNII: PDC6A3C0OX) THYMOL (UNII: 3J50XA376E) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83364-012-01 6 in 1 BOX 11/01/2024 1 3 g in 1 APPLICATOR; Type 0: Not a Combination Product 2 NDC:83364-012-02 12 in 1 BOX 11/01/2024 2 3 g in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 11/01/2024 Labeler - YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD (725220463)

