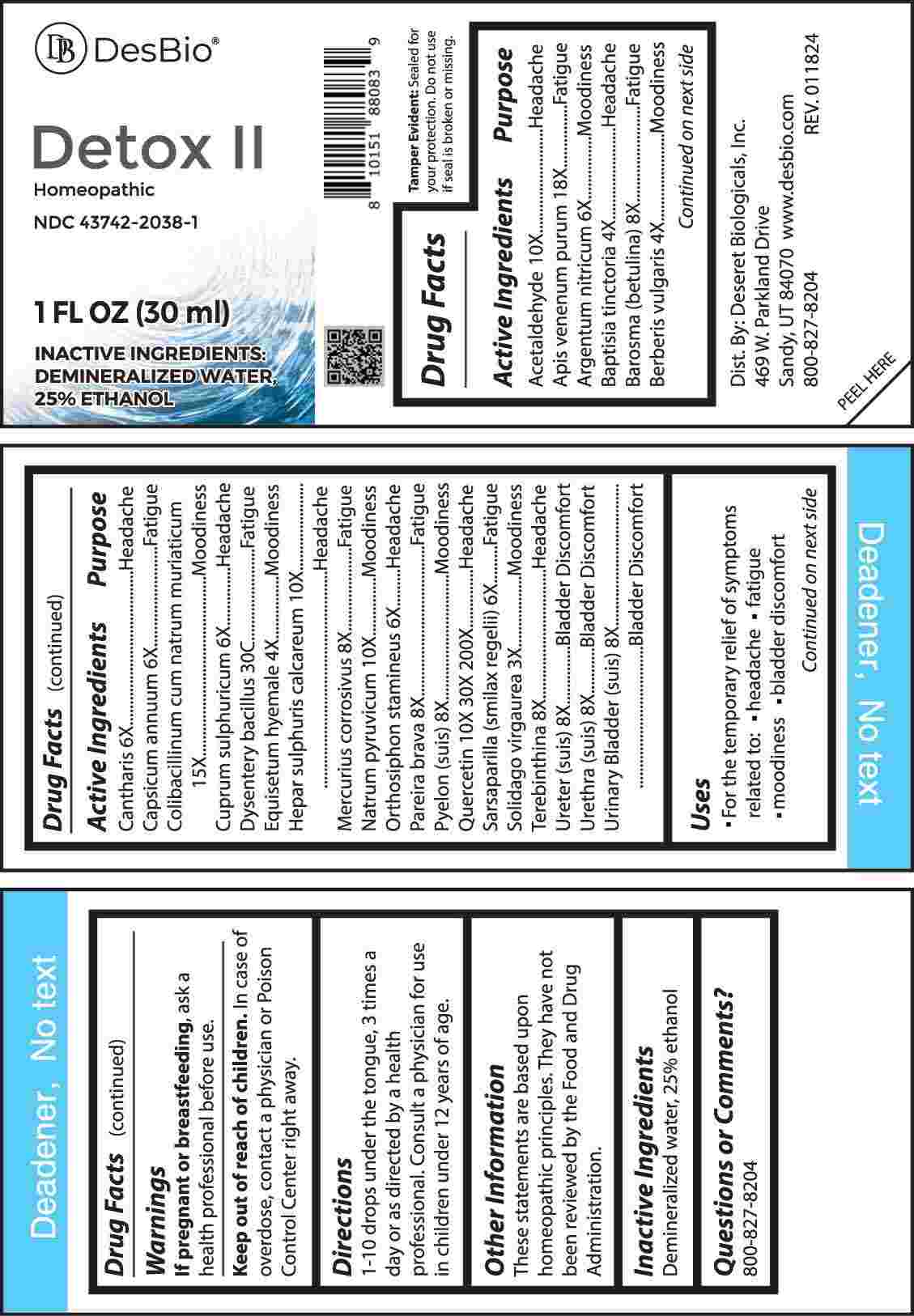
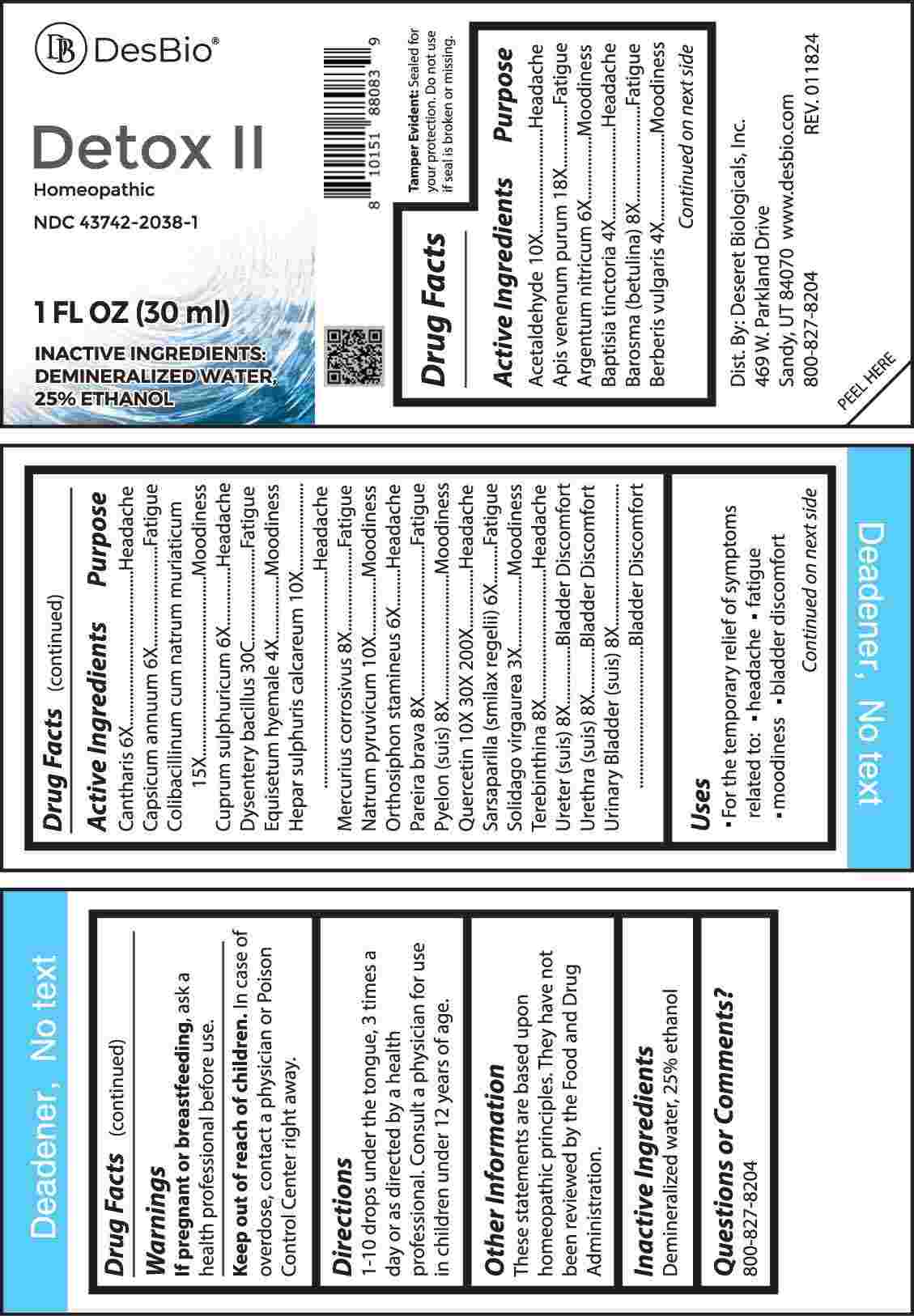
Label: DETOX II (solidago virgaurea, baptisia tinctoria, berberis vulgaris, equisetum hyemale, argentum nitricum, cantharis, capsicum annuum, cuprum sulphuricum, orthosiphon stamineus, sarsaparilla (smilax regelii), barosma (betulina), mercurius corrosivus, pareira brava, pyelon (suis), terebinthina, ureter (suis), urethra (suis), urinary bladder- suis, acetaldehyde, hepar sulphuris calcareum, natrum pyruvicum, quercetin, colibacillinum cum natrum muriaticum, apis venenum purum, dysentery bacillus liquid
- NDC Code(s): 43742-2038-1
- Packager: Deseret Biologicals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS:
Acetaldehyde 10X, Apis Venenum Purum 18X, Argentum Nitricum 6X, Baptisia Tinctoria 4X, Barosma (Betulina) 8X, Berberis Vulgaris 4X, Cantharis 6X, Capsicum Annuum 6X, Colibacillinum Cum Natrum Muriaticum 15X, Cuprum Sulphuricum 6X, Dysentery Bacillus 30C, Equisetum Hyemale 4X, Hepar Sulphuris Calcareum 10X, Mercurius Corrosivus 8X, Natrum Pyruvicum 10X, Orthosiphon Stamineus 6X, Pareira Brava 8X, Pyelon (Suis) 8X, Quercetin 10X, 30X, 200X, Sarsaparilla (Smilax Regelii) 6X, Solidago Virgaurea 3X, Terebinthina 8X, Ureter (Suis) 8X, Urethra (Suis) 8X, Urinary Bladder (Suis) 8X.
-
PURPOSE:
Acetaldehyde - Headache, Apis Venenum Purum - Fatigue, Argentum Nitricum - Moodiness, Baptisia Tinctoria - Headache, Barosma (Betulina) - Fatigue, Berberis Vulgaris - Moodiness, Cantharis - Headache, Capsicum Annuum - Fatigue, Colibacillinum Cum Natrum Muriaticum - Moodiness, Cuprum Sulphuricum - Headache, Dysentery Bacillus - Fatigue, Equisetum Hyemale - Moodiness, Hepar Sulphuris Calcareum - Headache, Mercurius Corrosivus - Fatigue, Natrum Pyruvicum - Moodiness, Orthosiphon Stamineus - Headache, Pareira Brava - Fatigue, Pyelon (Suis) – Moodiness, Quercetin - Headache, Sarsaparilla (Smilax Regelii) - Fatigue, Solidago Virgaurea – Moodiness, Terebinthina - Headache, Ureter (Suis) – Bladder Discomfort, Urethra (Suis) – Bladder Discomfort, Urinary Bladder (Suis) – Bladder Discomfort.
- USES:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
DETOX II
solidago virgaurea, baptisia tinctoria, berberis vulgaris, equisetum hyemale, argentum nitricum, cantharis, capsicum annuum, cuprum sulphuricum, orthosiphon stamineus, sarsaparilla (smilax regelii), barosma (betulina), mercurius corrosivus, pareira brava, pyelon (suis), terebinthina, ureter (suis), urethra (suis), urinary bladder (suis), acetaldehyde, hepar sulphuris calcareum, natrum pyruvicum, quercetin, colibacillinum cum natrum muriaticum, apis venenum purum, dysentery bacillus liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43742-2038 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOLIDAGO VIRGAUREA FLOWERING TOP (UNII: 5405K23S50) (SOLIDAGO VIRGAUREA FLOWERING TOP - UNII:5405K23S50) SOLIDAGO VIRGAUREA FLOWERING TOP 3 [hp_X] in 1 mL BAPTISIA TINCTORIA ROOT (UNII: 5EF0HWI5WU) (BAPTISIA TINCTORIA ROOT - UNII:5EF0HWI5WU) BAPTISIA TINCTORIA ROOT 4 [hp_X] in 1 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 4 [hp_X] in 1 mL EQUISETUM HYEMALE WHOLE (UNII: 59677RXH25) (EQUISETUM HYEMALE - UNII:59677RXH25) EQUISETUM HYEMALE WHOLE 4 [hp_X] in 1 mL SILVER NITRATE (UNII: 95IT3W8JZE) (SILVER CATION - UNII:57N7B0K90A) SILVER NITRATE 6 [hp_X] in 1 mL LYTTA VESICATORIA (UNII: 3Q034RO3BT) (LYTTA VESICATORIA - UNII:3Q034RO3BT) LYTTA VESICATORIA 6 [hp_X] in 1 mL CAPSICUM (UNII: 00UK7646FG) (CAPSICUM - UNII:00UK7646FG) CAPSICUM 6 [hp_X] in 1 mL CUPRIC SULFATE (UNII: LRX7AJ16DT) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 6 [hp_X] in 1 mL ORTHOSIPHON ARISTATUS WHOLE (UNII: 9091B04H7M) (ORTHOSIPHON ARISTATUS WHOLE - UNII:9091B04H7M) ORTHOSIPHON ARISTATUS WHOLE 6 [hp_X] in 1 mL SMILAX ORNATA ROOT (UNII: 2H1576D5WG) (SARSAPARILLA - UNII:2H1576D5WG) SMILAX ORNATA ROOT 6 [hp_X] in 1 mL AGATHOSMA BETULINA LEAF (UNII: 369DDH39Z0) (AGATHOSMA BETULINA LEAF - UNII:369DDH39Z0) AGATHOSMA BETULINA LEAF 8 [hp_X] in 1 mL MERCURIC CHLORIDE (UNII: 53GH7MZT1R) (MERCURIC CATION - UNII:ED30FJ8Y42) MERCURIC CHLORIDE 8 [hp_X] in 1 mL CHONDRODENDRON TOMENTOSUM ROOT (UNII: 395A3P448Z) (CHONDRODENDRON TOMENTOSUM ROOT - UNII:395A3P448Z) CHONDRODENDRON TOMENTOSUM ROOT 8 [hp_X] in 1 mL SUS SCROFA RENAL PELVIS (UNII: CH3YJ6606V) (SUS SCROFA RENAL PELVIS - UNII:CH3YJ6606V) SUS SCROFA RENAL PELVIS 8 [hp_X] in 1 mL TURPENTINE OIL (UNII: C5H0QJ6V7F) (TURPENTINE OIL - UNII:C5H0QJ6V7F) TURPENTINE OIL 8 [hp_X] in 1 mL SUS SCROFA URETER (UNII: 7PGE2DJ8QM) (SUS SCROFA URETER - UNII:7PGE2DJ8QM) SUS SCROFA URETER 8 [hp_X] in 1 mL SUS SCROFA URETHRA (UNII: 799ZL63XM1) (SUS SCROFA URETHRA - UNII:799ZL63XM1) SUS SCROFA URETHRA 8 [hp_X] in 1 mL SUS SCROFA URINARY BLADDER (UNII: 3G7U72W8DA) (SUS SCROFA URINARY BLADDER - UNII:3G7U72W8DA) SUS SCROFA URINARY BLADDER 8 [hp_X] in 1 mL ACETALDEHYDE (UNII: GO1N1ZPR3B) (ACETALDEHYDE - UNII:GO1N1ZPR3B) ACETALDEHYDE 10 [hp_X] in 1 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 10 [hp_X] in 1 mL SODIUM PYRUVATE (UNII: POD38AIF08) (PYRUVIC ACID - UNII:8558G7RUTR) SODIUM PYRUVATE 10 [hp_X] in 1 mL QUERCETIN (UNII: 9IKM0I5T1E) (QUERCETIN - UNII:9IKM0I5T1E) QUERCETIN 10 [hp_X] in 1 mL ESCHERICHIA COLI (UNII: 514B9K0L10) (ESCHERICHIA COLI - UNII:514B9K0L10) ESCHERICHIA COLI 15 [hp_X] in 1 mL APIS MELLIFERA VENOM (UNII: 76013O881M) (APIS MELLIFERA VENOM - UNII:76013O881M) APIS MELLIFERA VENOM 18 [hp_X] in 1 mL SHIGELLA DYSENTERIAE (UNII: 1EP6R5562J) (SHIGELLA DYSENTERIAE - UNII:1EP6R5562J) SHIGELLA DYSENTERIAE 30 [hp_C] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43742-2038-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 10/04/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/04/2021 Labeler - Deseret Biologicals, Inc. (940741853) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(43742-2038) , api manufacture(43742-2038) , label(43742-2038) , pack(43742-2038)