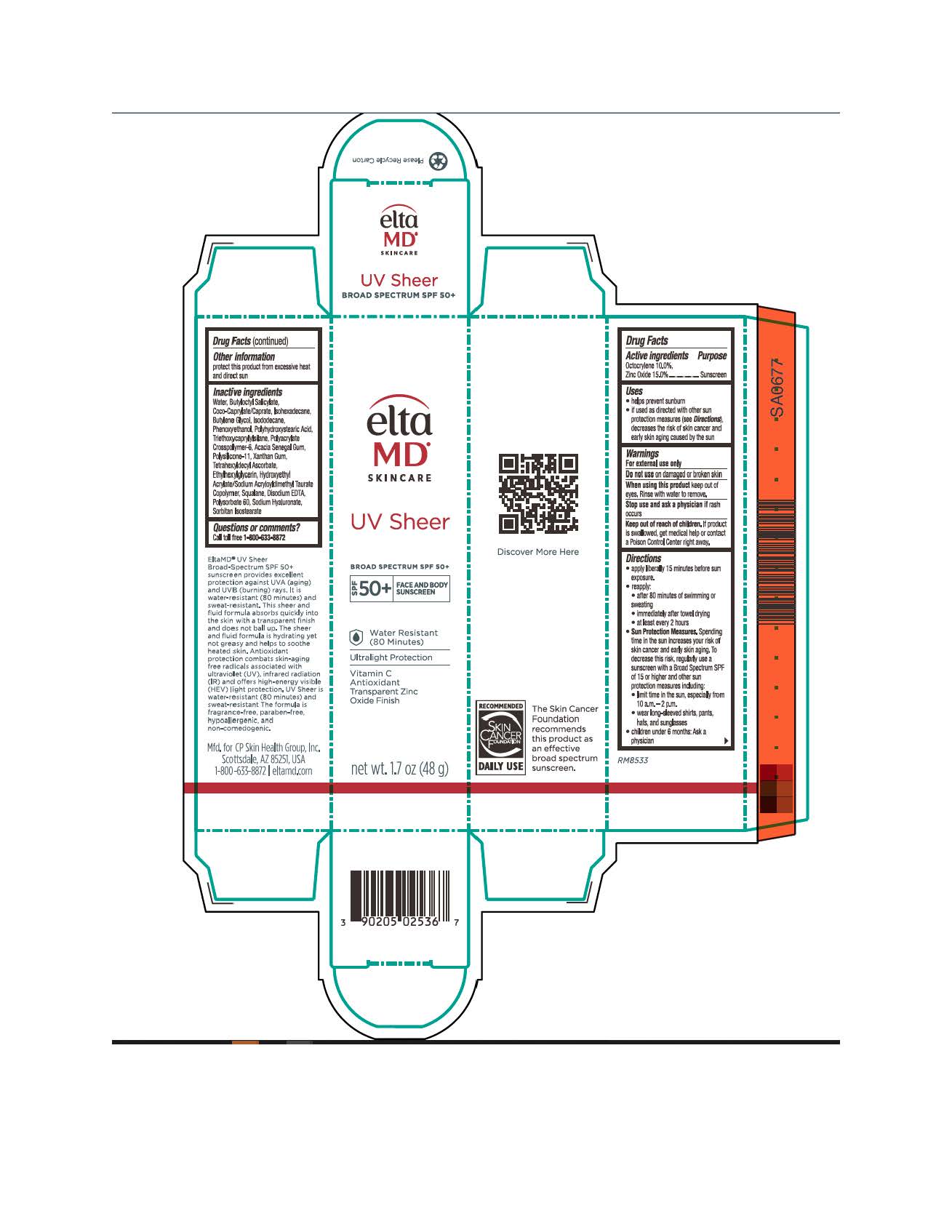
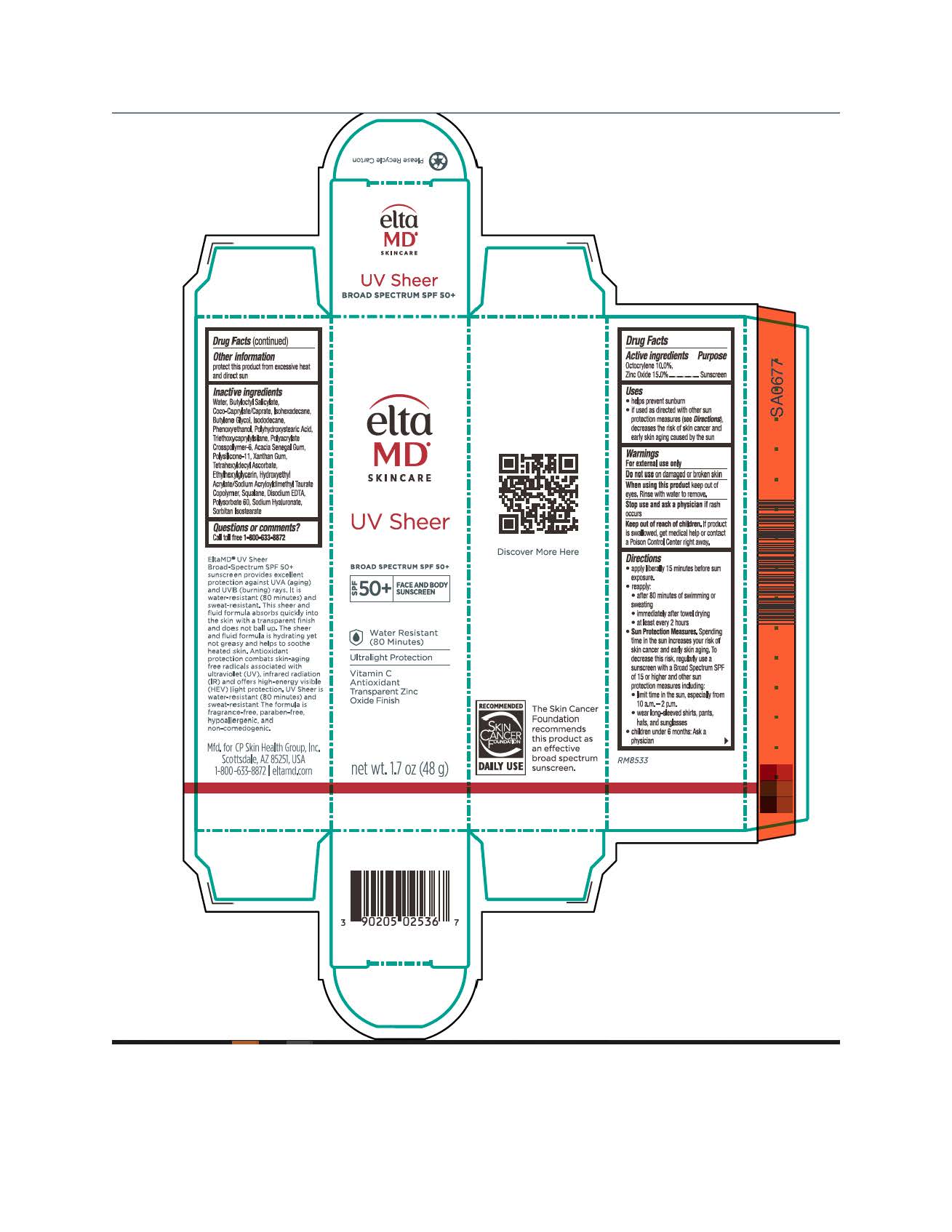
Label: ELTAMD UV SHEER- zinc oxide and octocrylene sunscreen cream
-
NDC Code(s):
72043-2563-0,
72043-2563-1,
72043-2563-2,
72043-2563-3, view more72043-2563-7
- Packager: CP Skin Health Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Warnings
- Active Ingredients
- Uses
- Uses
- Keep out of reach of children
-
Directions
Apply liberally 15 minutes before sun exposure. Reapply after 80 minutes of swimming or sweating, immediately after towel drying, at least every 2 hours. Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 am to 2 pm. Wear long-sleeve shirts, pants, hats, and sunglasses. Children under 6 months: ask a physician
- Other Information
-
Inactive ingredients
water, butyloctyl salicylate, coco-caprylate/caprate, isohexadecane, butylene glycol, isododecane, phenoxyethanol, polyhydroxystearic acid, triethoxycaprylylsilane, polyacrylate crosspolymer-6, acacia Senegal gum, polysilicone-11, xanthan gum, tetrahexyldecyl ascorbate, ethylhexylglycerin, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, squalene, disodium EDTA, polysorbate 60, sodium hyaluronate, sodium hydroxide
- Questions?
- Labeling
-
INGREDIENTS AND APPEARANCE
ELTAMD UV SHEER
zinc oxide and octocrylene sunscreen creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72043-2563 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 150 g in 1000 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 g in 1000 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYALURONIC ACID (UNII: S270N0TRQY) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM HYDROXIDE (UNII: 55X04QC32I) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ISODODECANE (UNII: A8289P68Y2) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ACACIA SENEGAL FLOWER (UNII: 72P931MTC2) XANTHAN GUM (UNII: TTV12P4NEE) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) EDETATE DISODIUM (UNII: 7FLD91C86K) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) SQUALANE (UNII: GW89575KF9) POLYSORBATE 60 (UNII: CAL22UVI4M) ISOHEXADECANE (UNII: 918X1OUF1E) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72043-2563-1 30 g in 1 TUBE; Type 0: Not a Combination Product 11/03/2020 11/09/2023 2 NDC:72043-2563-7 50 g in 1 TUBE; Type 0: Not a Combination Product 11/03/2020 3 NDC:72043-2563-0 10 g in 1 TUBE; Type 0: Not a Combination Product 11/03/2020 4 NDC:72043-2563-2 2 g in 1 PACKET; Type 0: Not a Combination Product 11/03/2020 11/09/2023 5 NDC:72043-2563-3 85 g in 1 TUBE; Type 0: Not a Combination Product 07/07/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/30/2020 Labeler - CP Skin Health Group, Inc. (611921669) Registrant - Swiss-American CDMO, LLC (080170933) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO, LLC 080170933 manufacture(72043-2563)