Label: CLEAR AS DAY SPF46- avobenzone, homosalate, octisalate, octocrylene gel
- NDC Code(s): 83171-001-01
- Packager: Starface World, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
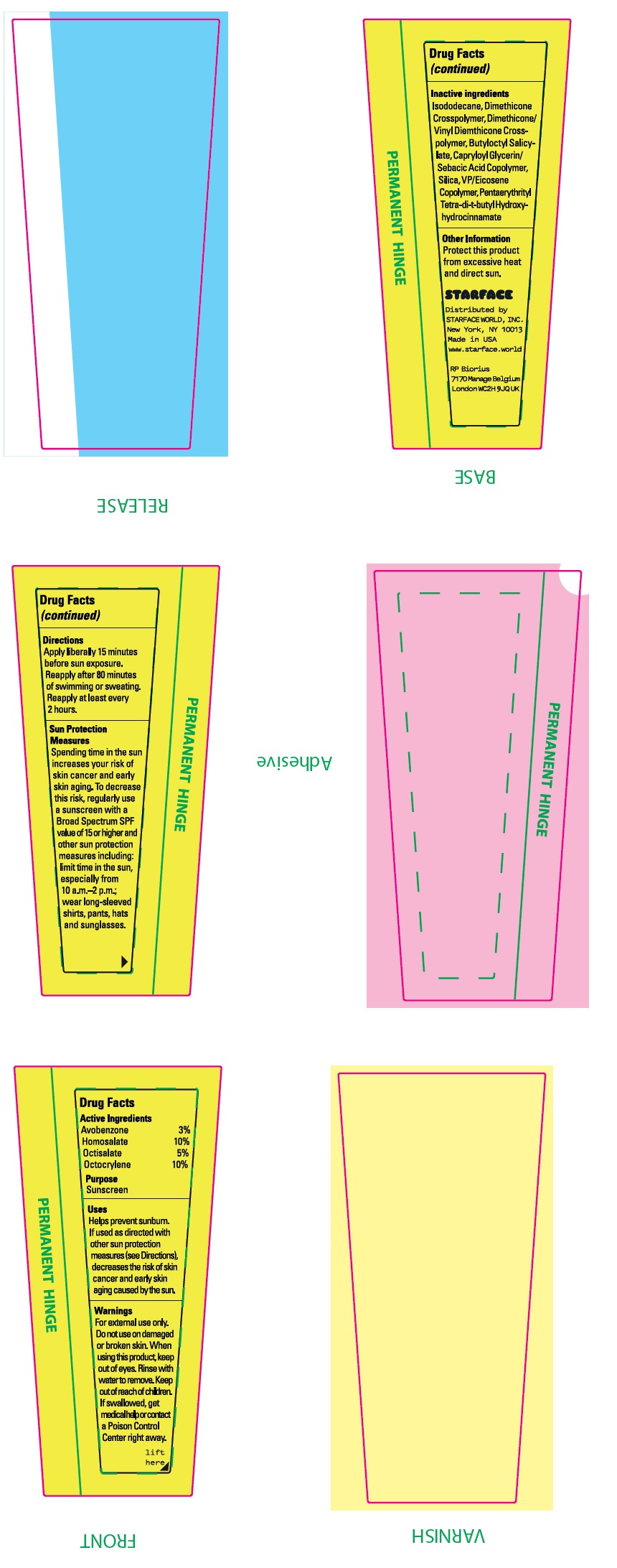
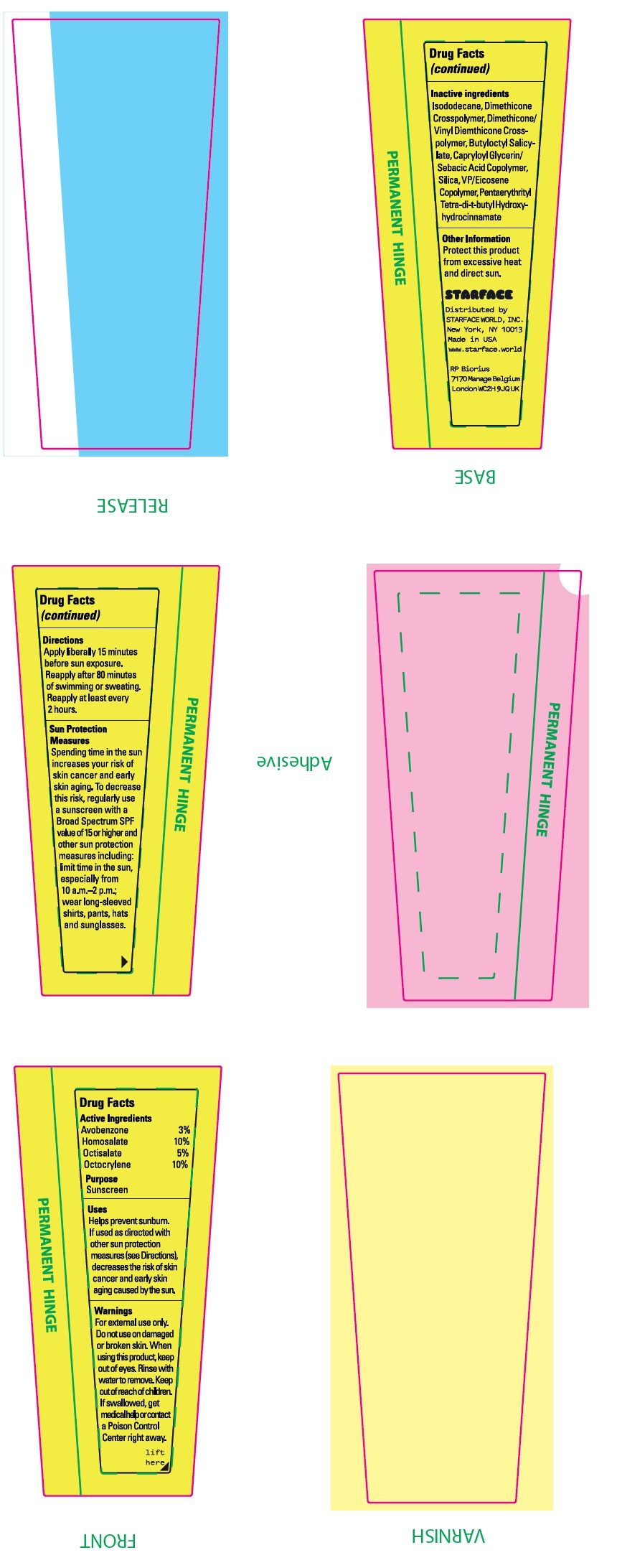
- Drug Facts
- Active Ingredients
- Uses:
- Warnings:
-
Directions:
Apply liberally 15 minutes before sun exposure. Reapply after 80 minutes of swimming or sweating. Reapply at least every 2 hours.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risl, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m.; wear long-sleeved shirts, pants, hats and sunglasses. Sun Protection Measures:
- Inactive ingredients:
- Other Information:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
CLEAR AS DAY SPF46
avobenzone, homosalate, octisalate, octocrylene gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83171-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) ISODODECANE (UNII: A8289P68Y2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83171-001-01 1 in 1 BOX 06/02/2022 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/02/2022 Labeler - Starface World, Inc. (040399707)