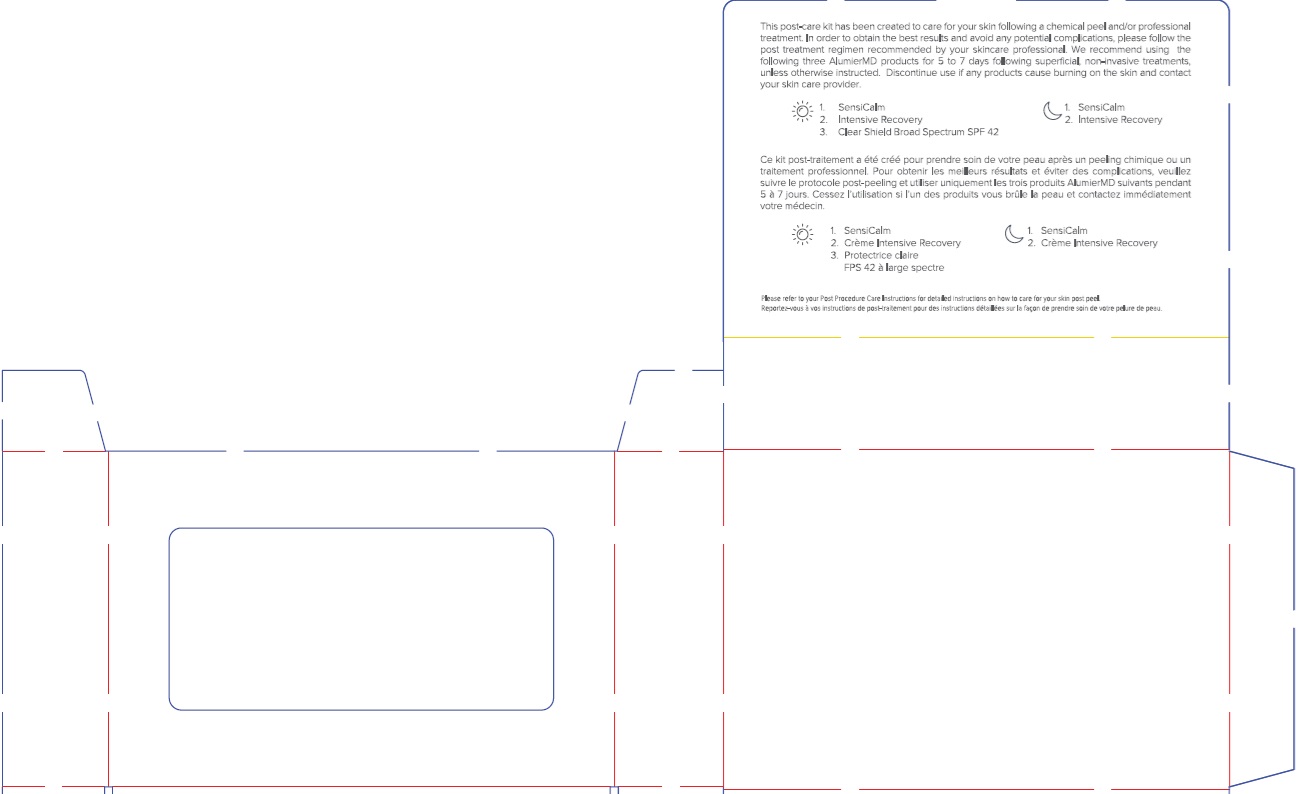
Label: ALUMIER MD POST PROCEDURE KIT WITH HYDROCORTISONE- titanium dioxide, zinc oxide, hydrocortisone kit
- NDC Code(s): 69473-020-01
- Packager: Alumier Labs
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Product Facts
- Active Ingredients
- Purpose
-
Uses
Clear Shield:
- helps prevent sunburn
Intensive Recovery:
- adults and children 2 years of age and older: For the temporary relief of minor skin irritations, rashes, itching and redness due to eczema, insect bites, poison ivy, poison oak, poison sumac, contact dermatitis (e.g. caused by soaps, detergents, cosmetics and/or jewelry), seborrheic dermatitis, psoriasis.
-
Warnings
For external use only
Do not use
Clear Shield:
- on broken skin
Intensive Recovery:
- for the treatment of diaper rash, except on the advice of a physician
When using this product
Clear Shield:
- avoid contact with eyes. If contact occurs, rinse thoroughly with water.
Intensive Recovery:
- avoid contact with eyes, do not use in or around the eyes
-
Directions
Clear Shield:
- apply liberally/generously (and evenly) 15 minutes before sun exposure
- reapply at least every 2 hours
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: - limit time in the sun, especially from 10 a.m. - 2 p.m. - wear long-sleeved shirts, pants, hats and sunglasses
- use a water resistant sunscreen if swimming or sweating
Intensive Recovery:
- adults and children 2 years of age and older, apply sparingly to affected area not more than three to four times daily
- do not use on children under 2 years of age, consult a physician
-
Other Information
Clear Shield:
- Store in a cool, dry place at room temperature.
- Do not use if security seal on box is broken or missing.
- You may report a serious adverse reaction event from using this product to: Alumier Labs Inc., 945 Concord Street, Framingham, MA 01701
Intensive Recovery:
- Do not use if security seal on box is broken or missing.
- You may report a serious adverse reaction event from using this product to: Alumier Labs Inc., 945 Concord Street, Framingham, MA 01701
-
Non-medicinal ingredients
Clear Shield:
Water/Aqua/Eau, C12-15 Alkyl Benzoate, Caprylic/Capric Triglyceride, Niacinamide, Propanediol, Dimethicone, Butyloctyl Salicylate, Glycerin, Caprylyl Methicone, Cetearyl Alcohol, Aluminium Stearate, Glyceryl Stearate, PEG-100 Stearate, Vitis Vinifera Seed Extract, Sodium Hyaluronate, Resveratrol, Lecithin, Magnesium Ascorbyl Phosphate, Tocopheryl Acetate, Glutathione, Acacia Senegal Gum, Cellulose Gum, Microcrystalline Cellulose, Polysilicone-11, Triethoxycaprylylsilane, Ceteareth-20, Polyhydroxystearic Acid, Xanthan Gum, Disodium EDTA, Alumina, Tocopherol, Alteromonas Ferment Extract, Butylene Glycol, Physalis Angulata Extract, Polygonum Aviculare Extract, Xylitol, Caprylic Acid, Caprylyl Glycol, Polymethyl Methacrylate, Tricaprylin, Sodium Benzoate, Potassium Sorbate, Pentylene Glycol, Ethylhexylglycerin.
Intensive Recovery:
Water/Aqua/Eau, Butyrospermum Parkii (Shea Butter), Simmondsia Chinensis (Jojoba) Seed Oil, Glycerin, Caprylic/Capric Triglyceride, Dimethicone, Propanediol, Theobroama Cacao (Cocoa) Seed Butter, Aloe Barbadensis Leaf Juice, Niacinamide, Resveratrol, Poria Cocos Sclerotium Extract, Boswellia Serrata Gum Extract, Honey Extract (Extait De. Miel), Tetrapeptide-14, Copper Tripeptide-1, Aspalathus Linearis Extract, Ceramide NP, Ceramide NG, Squalane, Hydrogenated Lecithin, Sodium Acrylates Copolymer, Lecithin, Behenyl Alchol, Xanthan Gum, Caprylic Acid, Sodium Methyl Stearoyl Taurate, Tricaprylin, Polymethyl Methacrylate, Sodium Hydroxide, Disodium EDTA, Caprylyl Glycol, Xylitol, Ethylhexylglycerin.
- Product Packaging
-
INGREDIENTS AND APPEARANCE
ALUMIER MD POST PROCEDURE KIT WITH HYDROCORTISONE
titanium dioxide, zinc oxide, hydrocortisone kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69473-020 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69473-020-01 1 in 1 CARTON; Type 0: Not a Combination Product 10/25/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 15 mL Part 2 1 TUBE 15 mL Part 3 1 TUBE 15 mL Part 1 of 3 ALUMIER MD CLEAR SHIELD BROAD SPECTRUM SPF 42 SUNSCREEN
titanium dioxide, zinc oxide lotionProduct Information Item Code (Source) NDC:69473-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 61.8 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 70 mg in 1 mL Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) CAPRYLIC ACID (UNII: OBL58JN025) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYALURONATE SODIUM (UNII: YSE9PPT4TH) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TRICAPRYLIN (UNII: 6P92858988) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) NIACINAMIDE (UNII: 25X51I8RD4) CAPRYLYL METHICONE (UNII: Q95M2P1KJL) POLYGONUM AVICULARE TOP (UNII: ZCD6009IUF) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) WATER (UNII: 059QF0KO0R) ACACIA (UNII: 5C5403N26O) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) GLYCERIN (UNII: PDC6A3C0OX) VITIS VINIFERA SEED (UNII: C34U15ICXA) ALUMINUM MONOSTEARATE (UNII: P9BC99461E) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) XANTHAN GUM (UNII: TTV12P4NEE) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PROPANEDIOL (UNII: 5965N8W85T) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) EDETATE DISODIUM (UNII: 7FLD91C86K) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PEG-100 STEARATE (UNII: YD01N1999R) ALUMINUM OXIDE (UNII: LMI26O6933) GLUTATHIONE (UNII: GAN16C9B8O) PHENOXYETHANOL (UNII: HIE492ZZ3T) RESVERATROL (UNII: Q369O8926L) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TOCOPHEROL (UNII: R0ZB2556P8) PHYSALIS ANGULATA (UNII: W4TKW9D5GG) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) SODIUM BENZOATE (UNII: OJ245FE5EU) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PENTYLENE GLYCOL (UNII: 50C1307PZG) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 15 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/23/2024 Part 2 of 3 ALUMIER MD SENSICALM GENTLE FACIAL CLEANSER
cleansing (cold creams, cleansing lotions, liquids, and pads) [skin care preparations (creams, lotions, powder, and sprays)] creamProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR LINALOOL, (+/-)- (UNII: D81QY6I88E) INGR SORBITAN MONOOLEATE (UNII: 06XEA2VD56) INGR CERAMIDE NG (UNII: C04977SRJ5) INGR POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) INGR CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) INGR SUCROSE STEARATE (UNII: 274KW0O50M) INGR CETYL ALCOHOL (UNII: 936JST6JCN) INGR NIACINAMIDE (UNII: 25X51I8RD4) INGR ALLANTOIN (UNII: 344S277G0Z) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR P-ANISIC ACID (UNII: 4SB6Y7DMM3) INGR XANTHAN GUM (UNII: TTV12P4NEE) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) INGR XYLITOL (UNII: VCQ006KQ1E) INGR CAPRYLIC ACID (UNII: OBL58JN025) INGR 4-TERPINEOL, (+/-)- (UNII: L65MV77ZG6) INGR EDETATE SODIUM (UNII: MP1J8420LU) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR COCAMIDOPROPYL HYDROXYSULTAINE (UNII: 62V75NI93W) INGR WATER (UNII: 059QF0KO0R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR PROPANEDIOL (UNII: 5965N8W85T) INGR SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) INGR ALOE VERA LEAF (UNII: ZY81Z83H0X) INGR YUCCA SCHIDIGERA ROOT (UNII: E2H9ET15AT) INGR SUNFLOWER OIL (UNII: 3W1JG795YI) INGR LINALYL ACETATE (UNII: 5K47SSQ51G) INGR .BETA.-OCIMENE, (3Z)- (UNII: 472UVP4R7T) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR DISODIUM COCOYL GLUTAMATE (UNII: MBK0CP8F5A) INGR SODIUM COCOYL GLUTAMATE (UNII: BMT4RCZ3HG) INGR ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 15 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 10/23/2024 Part 3 of 3 ALUMIER MD INTENSIVE RECOVERY
hydrocortisone creamProduct Information Item Code (Source) NDC:69473-017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) DOCOSANOL (UNII: 9G1OE216XY) COCOA BUTTER (UNII: 512OYT1CRR) NIACINAMIDE (UNII: 25X51I8RD4) PREZATIDE COPPER (UNII: 6BJQ43T1I9) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) HONEY (UNII: Y9H1V576FH) EDETATE DISODIUM (UNII: 7FLD91C86K) TRICAPRYLIN (UNII: 6P92858988) XYLITOL (UNII: VCQ006KQ1E) SODIUM METHYL STEAROYL TAURATE (UNII: JFM219LJ55) XANTHAN GUM (UNII: TTV12P4NEE) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPANEDIOL (UNII: 5965N8W85T) SHEA BUTTER (UNII: K49155WL9Y) JOJOBA OIL (UNII: 724GKU717M) RESVERATROL (UNII: Q369O8926L) ASPALATHUS LINEARIS LEAF (UNII: H7UGK1GJCU) SQUALANE (UNII: GW89575KF9) ALOE VERA LEAF (UNII: ZY81Z83H0X) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) SODIUM HYDROXIDE (UNII: 55X04QC32I) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CAPRYLIC ACID (UNII: OBL58JN025) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) CERAMIDE NP (UNII: 4370DF050B) CERAMIDE NG (UNII: C04977SRJ5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 15 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/25/2024 Labeler - Alumier Labs (079603173)