Label: BABYGANICS DIAPER RASH- zinc oxide cream
- NDC Code(s): 59062-1219-1
- Packager: KAS Direct LLC dba BabyGanics
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 4, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
water, butyrospermum parkii (shea) butter, theobroma cacao (cocoa) seed butter, prunus amygdalus dulcis (sweet almond) oil, glycol stearate, simmondsia chinensis (jojoba) seed oil, polyglyceryl-3 polyricinoleate, glycerin, tocopheryl acetate, solanum lycopersicum (tomato) seed oil, helianthus annuus (sunflower) seed oil, vaccinium macrocarpon (cranberry) seed oil, nigella sativa (black cumin) seed oil, rubus idaeus (red raspberry) seed oil, calendula officinalis flower extract, aloe barbadensis leaf juice, sorbitan sesquioleate, cetyl ricinoleate, glyceryl caprate, beeswax, sodium polyacryloyldimethyl taurate, hydroxyethylcellulose, hydrogenated polydecene, trideceth-10, butylene glycol, magnesium stearate, aluminum tristearate, sodium benzoate, gluconolactone
- SPL UNCLASSIFIED SECTION
-
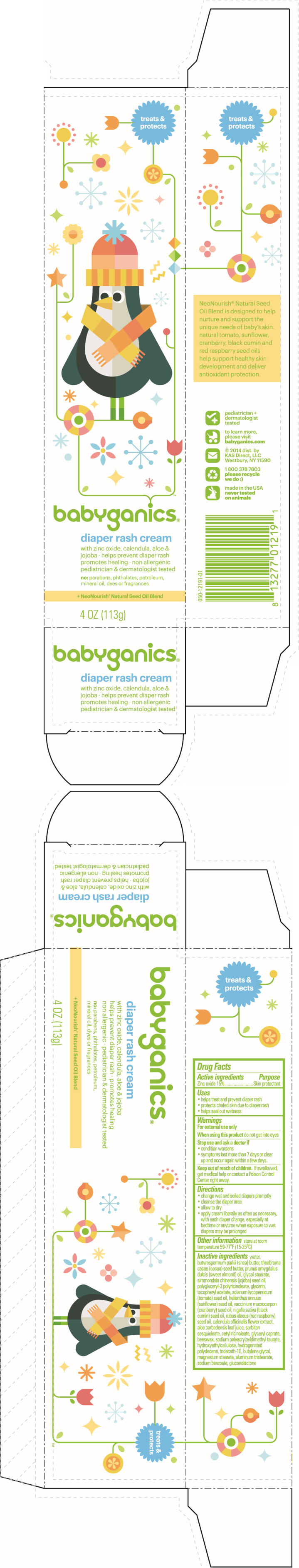
PRINCIPAL DISPLAY PANEL - 113 g Tube Carton
treats &
protectsbabyganics®
diaper rash cream
with zinc oxide, calendula, aloe &
jojoba ∙ helps prevent diaper rash
promotes healing ∙ non allergenic
pediatrician & dermatologist testedno: parabens, phthalates, petroleum,
mineral oil, dyes or fragrances+ NeoNourish® Natural Seed Oil Blend
4 OZ (113g)
-
INGREDIENTS AND APPEARANCE
BABYGANICS DIAPER RASH
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59062-1219 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 150 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SHEANUT OIL (UNII: O88E196QRF) GLYCOL STEARATE (UNII: 0324G66D0E) JOJOBA OIL (UNII: 724GKU717M) POLYGLYCERYL-3 RICINOLEATE (UNII: MZQ63P0N0W) GLYCERIN (UNII: PDC6A3C0OX) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOMATO SEED OIL (UNII: 7N87T9C06T) SUNFLOWER OIL (UNII: 3W1JG795YI) CRANBERRY SEED OIL (UNII: 73KDS3BW5E) NIGELLA SATIVA SEED OIL (UNII: CS4U38E731) RASPBERRY SEED OIL (UNII: 9S8867952A) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) ALOE VERA LEAF (UNII: ZY81Z83H0X) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) CETYL RICINOLEATE (UNII: 1P677500YD) GLYCERYL CAPRATE (UNII: 197M6VFC1W) YELLOW WAX (UNII: 2ZA36H0S2V) SODIUM TAURATE (UNII: Y7LJA74T0N) HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) HYDROGENATED POLYDECENE (550 MW) (UNII: U333RI6EB7) TRIDECETH-10 (UNII: G624N6MSBA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) MAGNESIUM STEARATE (UNII: 70097M6I30) ALUMINUM STEARATE (UNII: U6XF9NP8HM) SODIUM BENZOATE (UNII: OJ245FE5EU) GLUCONOLACTONE (UNII: WQ29KQ9POT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59062-1219-1 1 in 1 CARTON 06/21/2013 1 113 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part347 06/21/2013 Labeler - KAS Direct LLC dba BabyGanics (002764605)

