Label: BT PAIN RELIEF- menthol, methyl salicylate patch
- NDC Code(s): 82198-0011-1
- Packager: Big 5 Nutrition LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
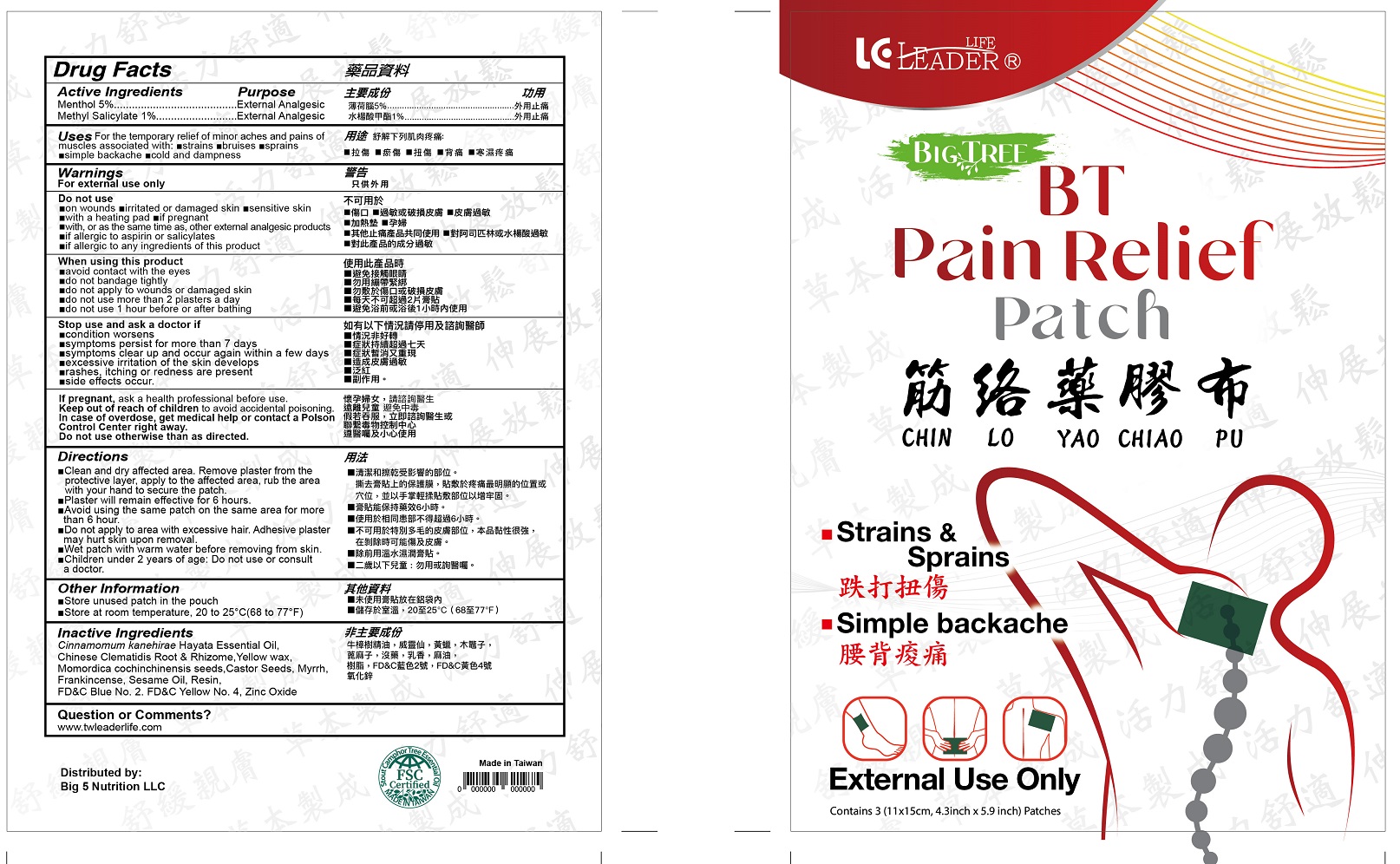
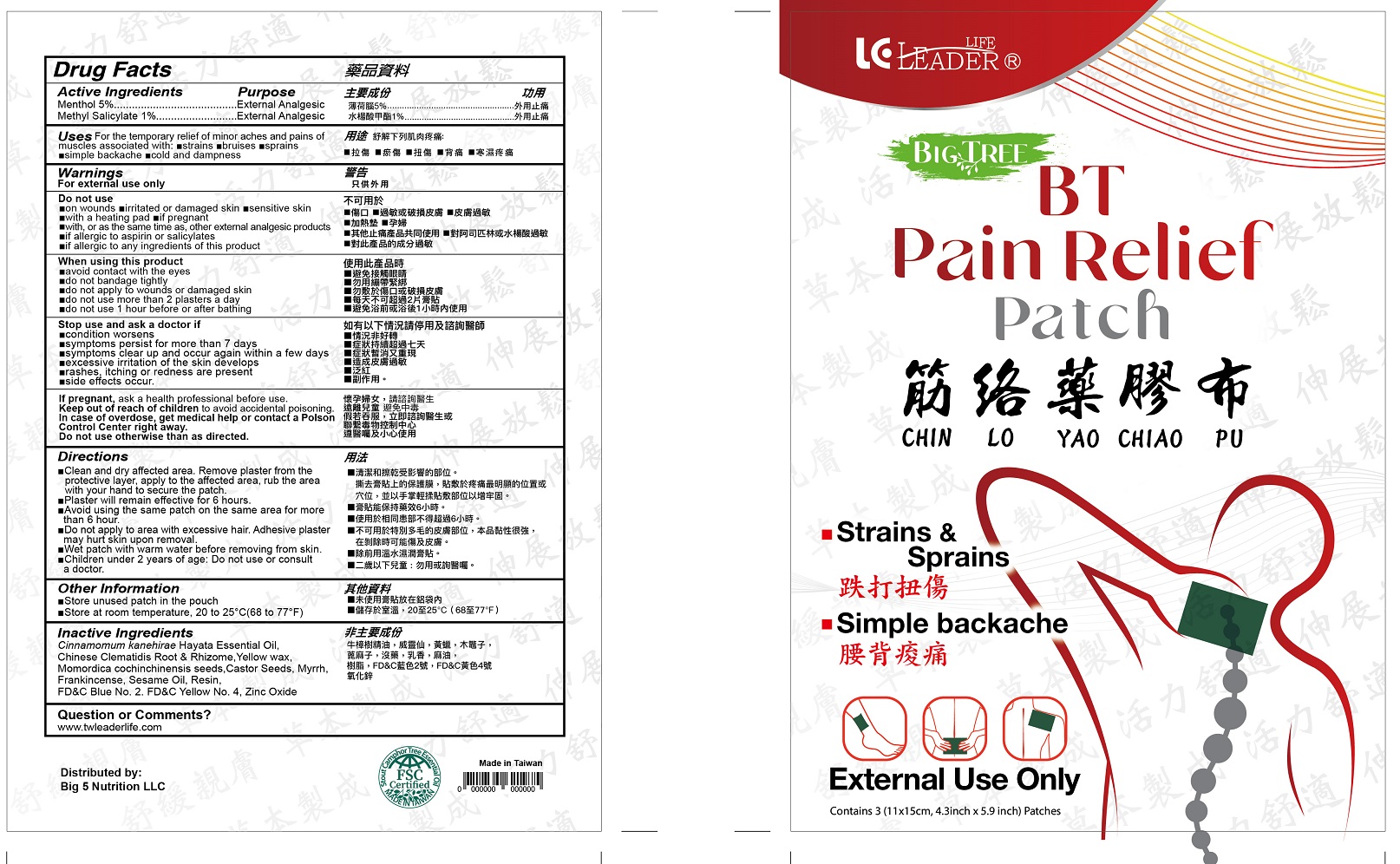
- Drug Facts
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only
Do not use
•on wounds •irritated or damaged skin •sensitive skin
•with a heating pad •if pregnant
•with, or as the same time as, other external analgesic products
•if allergic to aspirin or salicylates
•if allergic to any ingredients of this product
When using this product
•avoid contact with the eyes
•do not bandage tightly
•do not apply to wounds or damaged skin
•do not use more than 2 plasters a day
•do not use 1 hour before or after bathing
Stop use and ask a doctor if
•condition worsens
•symptoms persist for more than 7 days
•symptoms clear up and occur again within a few days
•excessive irritation of the skin develops
•rashes, itching or redness are present
•side effects occur
If pregnant, ask a health professional before use.
- KEEP OUT OF REACH OF CHILDREN
-
Directions
• Clean and dry affected area. Remove plaster from the protective layer, apply to the affected area, rub the area with your hand to secure the patch.
•Patch will remain effective for 6 hours.
•Avoid using the same patch on the same area for more than 6 hours.
•Do not apply to area with excessive hair. Adhesive plaster may hurt skin upon removal.
•Wet patch with warm water before removing from skin
•Children under 2 years of age: Do not use or consult a doctor. - Other information
- Inactive ingredients
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
BT PAIN RELIEF
menthol, methyl salicylate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82198-0011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 0.06 g in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength CLEMATIS CHINENSIS ROOT (UNII: 8Z18N528CU) INDIGOTINDISULFONATE SODIUM (UNII: D3741U8K7L) MOMORDICA COCHINCHINENSIS SEED (UNII: 2T87O1UPVD) MYRRH (UNII: JC71GJ1F3L) FRANKINCENSE (UNII: R9XLF1R1WM) PINUS MASSONIANA RESIN (UNII: 64S07U83T7) RICINUS COMMUNIS SEED (UNII: 7EK4SFN1TX) SESAME OIL (UNII: QX10HYY4QV) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) YELLOW WAX (UNII: 2ZA36H0S2V) ZINC OXIDE (UNII: SOI2LOH54Z) Product Characteristics Color green Score Shape RECTANGLE Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82198-0011-1 3 in 1 BAG 01/01/2024 1 5.9 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 01/01/2024 Labeler - Big 5 Nutrition LLC (114574559) Establishment Name Address ID/FEI Business Operations Taiwan Shuenn-Ann Biotechnology Pharmaceutical Co., Ltd. 656348265 manufacture(82198-0011) , label(82198-0011) , pack(82198-0011)