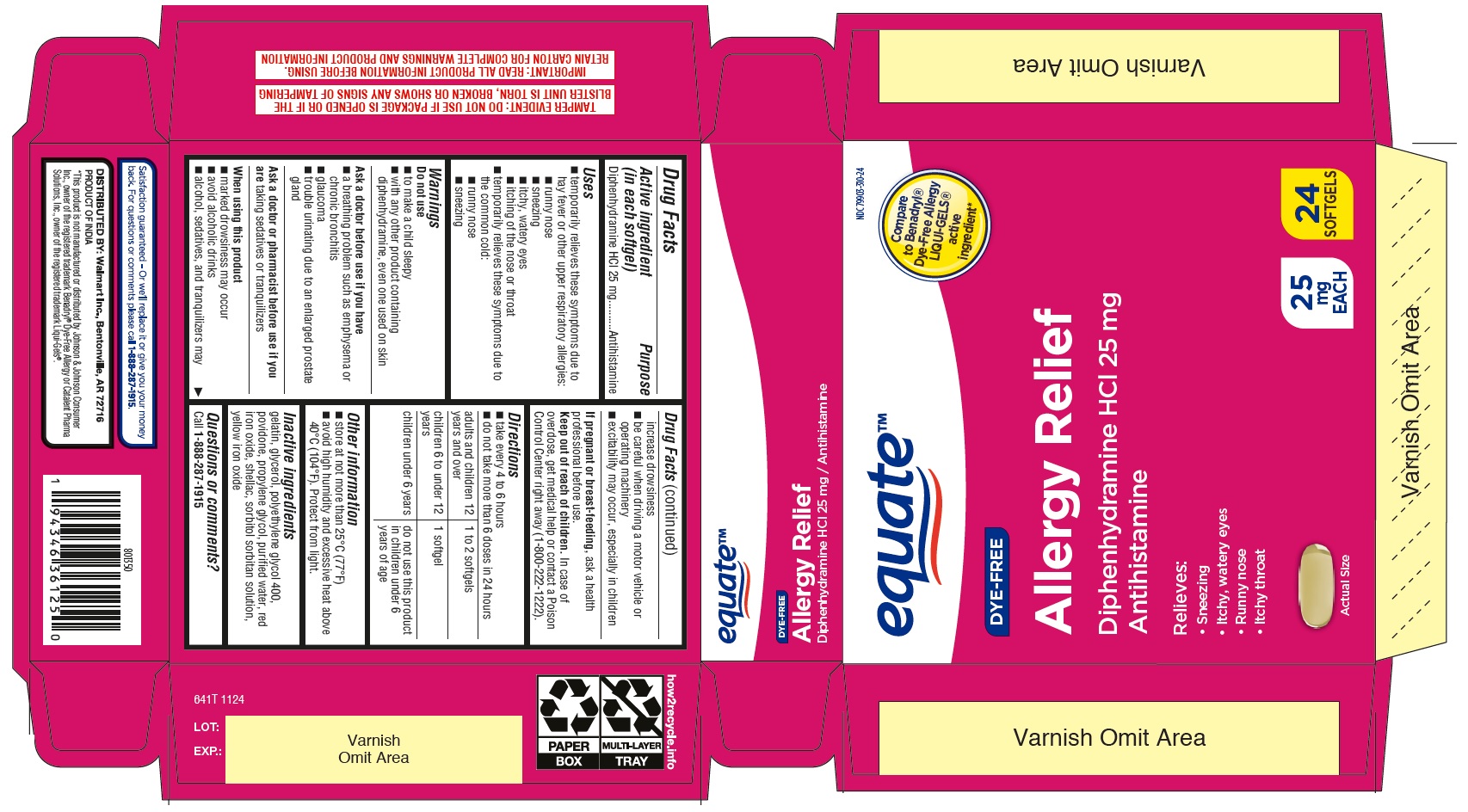
Label: ALLERGY RELIEF- diphenhydramine hydrochloride capsule, liquid filled
- NDC Code(s): 79903-310-24
- Packager: Walmart Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each softgel)
- Purpose
- Uses
- WARNINGS
- Do not use
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- INACTIVE INGREDIENT
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
diphenhydramine hydrochloride capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-310 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SHELLAC (UNII: 46N107B71O) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GLYCERIN (UNII: PDC6A3C0OX) POVIDONE (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) GELATIN (UNII: 2G86QN327L) FERRIC OXIDE RED (UNII: 1K09F3G675) SORBITOL SOLUTION (UNII: 8KW3E207O2) SORBITAN (UNII: 6O92ICV9RU) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Product Characteristics Color yellow (Light yellow to yellow) Score no score Shape CAPSULE (oblong) Size 15mm Flavor Imprint Code 157 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-310-24 2 in 1 CARTON 11/25/2024 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/25/2024 Labeler - Walmart Inc. (051957769) Registrant - TIME CAP LABORATORIES, INC. (037052099) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LIMITED 925822975 manufacture(79903-310)