Label: CLOTRIMAZOLE liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 0363-3492-01 - Packager: Walgreens
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 2, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
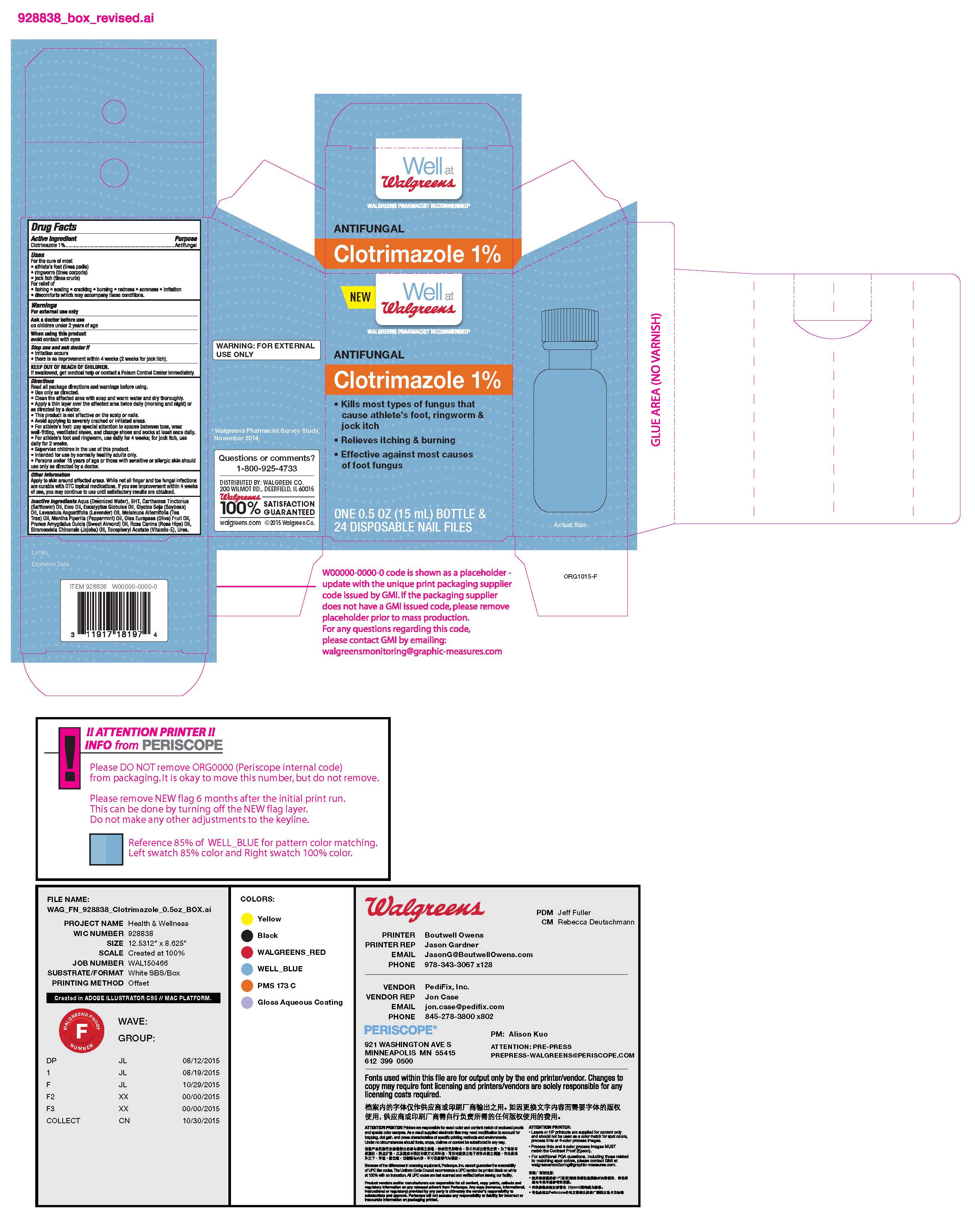
- ACTIVE INGREDIENT
- WARNINGS
- PURPOSE
-
DOSAGE & ADMINISTRATION
• Use only as directed.
• Clean the affected area with soap and warm water and dry thoroughly.
• Apply a thin layer over the affected area twice daily (morning and night) or as directed by a doctor.
• This product is not effective on the scalp or nails.
• Avoid applying to severely cracked or irritated areas.
• For athlete’s foot: pay special attention to spaces between toes, wear well-fit- ting, ventilated shoes, and change shoes and socks at least once daily.
• For athlete’s foot and ringworm, use daily for 4 weeks; for jock itch, use daily
for 2 weeks.
• Super vise children in the use of this product.
• Intended for use by normally healthy adults only.
• Persons under 18 years of age or those with sensitive or allergic skin should use only as directed by a doctor.
- KEEP OUT OF REACH OF CHILDREN
-
INACTIVE INGREDIENT
Aqua (Deionized Water), BHT, Car thamus Tinctorius (Saf flower) Oil, Emu Oil, Eucalyptus Globulus Oil, Glycine Soja (Soybean) Oil, Lavandula Angustifolia (Lavender) Oil, Melaleuca Alternifolia (Tea
Tree) Oil, Mentha Piperita (Peppermint) Oil, Olea Europaea (Olive) Fruit Oil, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Rosa Canina (Rose Hips) Oil, Simmondsia Chinensis (Jojoba) Oil, Tocopher yl Acetate (Vitamin- E), Urea.
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLOTRIMAZOLE
clotrimazole liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-3492 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOTRIMAZOLE (UNII: G07GZ97H65) (CLOTRIMAZOLE - UNII:G07GZ97H65) CLOTRIMAZOLE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARTHAMUS TINCTORIUS FLOWER OIL (UNII: SDQ136WIM5) MENTHA PIPERITA (UNII: 79M2M2UDA9) SIMMONDSIA CHINENSIS WHOLE (UNII: DFM16KFA82) EMU OIL (UNII: 344821WD61) OLEA EUROPAEA FRUIT VOLATILE OIL (UNII: 8E7358CX1J) ALMOND OIL (UNII: 66YXD4DKO9) UREA (UNII: 8W8T17847W) EUCALYPTUS GLOBULUS WHOLE (UNII: SI1P2XF3M3) ROSA CANINA FLOWER OIL (UNII: DUY7M48I1T) WATER (UNII: 059QF0KO0R) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) LAVENDER OIL (UNII: ZBP1YXW0H8) MELALEUCA ALTERNIFOLIA WHOLE (UNII: 976KV4FXYF) SOYBEAN OIL (UNII: 241ATL177A) .ALPHA.-TOCOPHEROL SUCCINATE, D- (UNII: LU4B53JYVE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-3492-01 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 11/01/2015 Labeler - Walgreens (008965063) Registrant - walgreens (008965063)