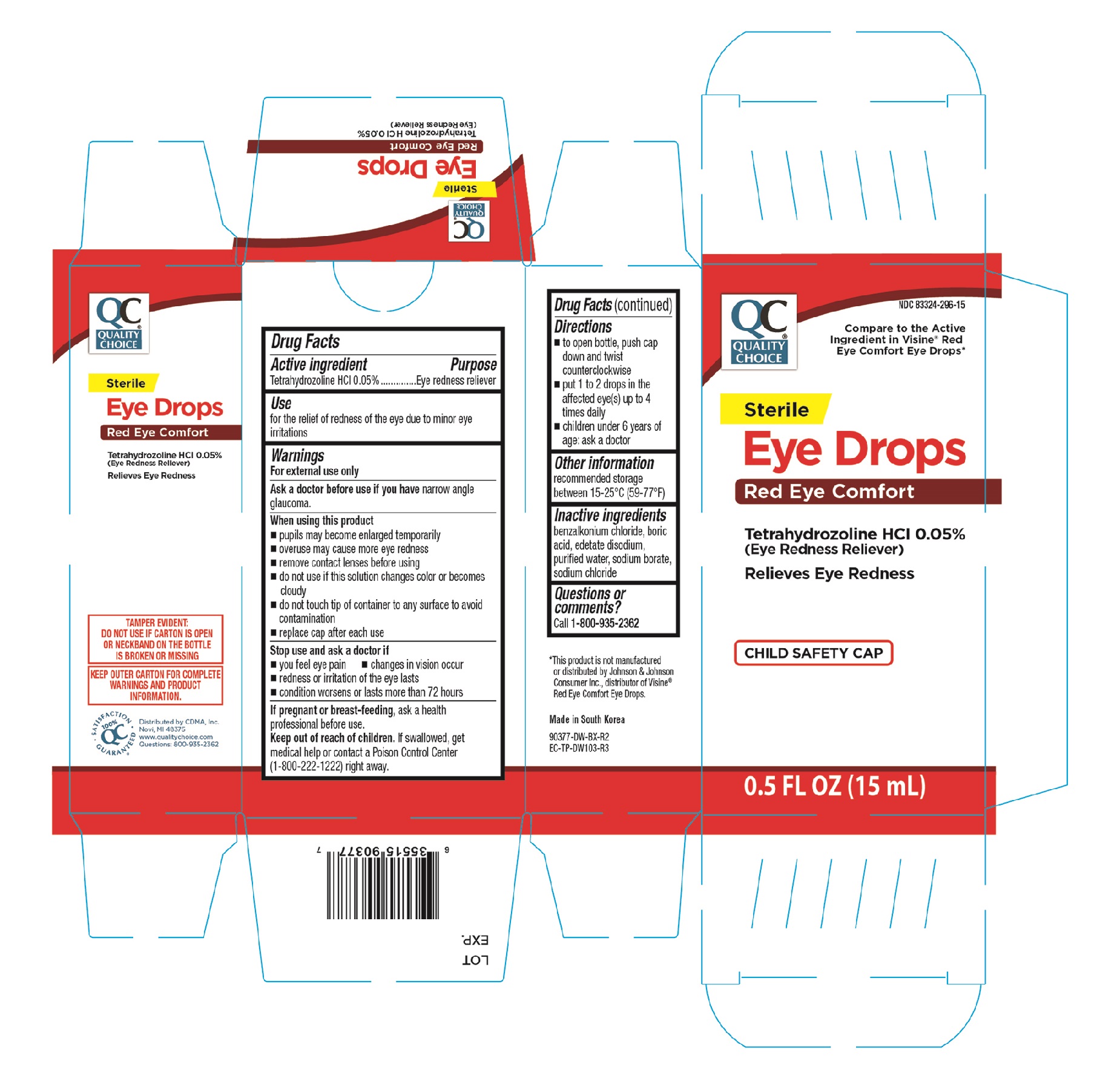
Label: QC STERILE RED EYE- tetrahydrozoline hci solution/ drops
- NDC Code(s): 83324-296-15
- Packager: Chain Drug Marketing Association, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- ASK DOCTOR
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
QC STERILE RED EYE
tetrahydrozoline hci solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83324-296 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TETRAHYDROZOLINE HYDROCHLORIDE (UNII: 0YZT43HS7D) (TETRAHYDROZOLINE - UNII:S9U025Y077) TETRAHYDROZOLINE HYDROCHLORIDE 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM BORATE (UNII: 91MBZ8H3QO) EDETATE DISODIUM (UNII: 7FLD91C86K) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) BORIC ACID (UNII: R57ZHV85D4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83324-296-15 1 in 1 CARTON 11/01/2024 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 08/01/2024 Labeler - Chain Drug Marketing Association, Inc. (011920774)