Label: HEMORRHOIDAL COOLING GEL- phenylephrine and witch hazel gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 49348-811-32 - Packager: McKesson
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 4, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USES
-
WARNINGS
For external use only.
Ask a doctor before use if you have
- heart disease
- thyroid disease
- high blood pressure
- diabetes
- difficulty in urination due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug for high blood pressure or depression.
-
DIRECTIONS
- Adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a tissue or a soft cloth before applying gel.
- When first opening the tube, puncture foil seal with top end of cap
- Apply externally to the affected area up to 4 times daily, especially at night, in the morning, or after each bowel movement
- SPL UNCLASSIFIED SECTION
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS
-
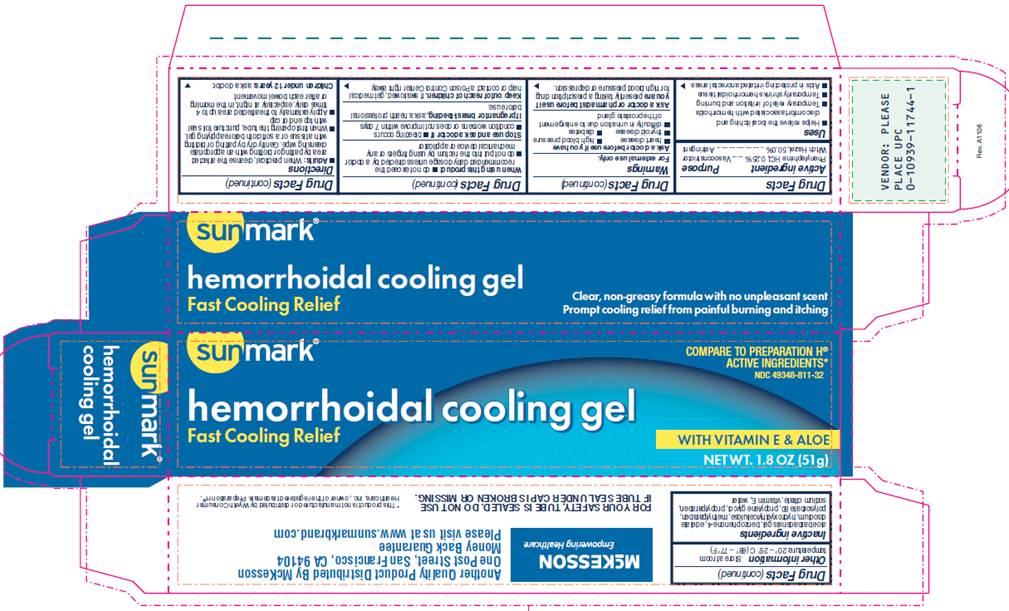
PACKAGE INFORMATION - TUBE
Sunmark®
COMPARE TO PREPARATION H® ACTIVE INGREDIENTS*
NDC 49348-811-32
Hemorrhoidal Cooling Gel
Fast Cooling Relief
WITH VITAMIN E AND ALOE
NET WT. 1.8 OZ (51 g)
This tube is sealed and packaged in a carton. Do not buy if carton is missing. Do not use if the seal under the cap has been punctured or if the tube is damaged.
Distributed by McKesson
One Post Street
San Francisco, CA 94104
Money Back Guarantee

-
PACKAGE INFORMATION - CARTON
Sunmark®
COMPARE TO PREPARATION H® ACTIVE INGREDIENTS*
NDC 49348-811-32
Hemorrhoidal Cooling Gel
Fast Cooling Relief
Clear, non-greasy formula with no unpleasant scent
Prompt cooling relief from painful burning and itching
WITH VITAMIN E AND ALOE
NET WT. 1.8 OZ (51 g)
Another Quality Product Distributed by McKesson
One Post Street, San Francisco, CA 94104
Money Back Guarantee
Please visit us at www.sunmarkbrand.com
FOR YOUR SAFETY, TUBE IS SEALED. DO NOT USE IF TUBE SEAL UNDER CAP IS BROKEN OR MISSING.
*This product is not manufactured by Wyeth Consumer Healthcare, Inc., owner of the registered trademark Preparation H®.
-
INGREDIENTS AND APPEARANCE
HEMORRHOIDAL COOLING GEL
phenylephrine and witch hazel gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49348-811 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 0.25 g in 1 g WITCH HAZEL (UNII: 101I4J0U34) (WITCH HAZEL - UNII:101I4J0U34) WITCH HAZEL 50 g in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) SULISOBENZONE (UNII: 1W6L629B4K) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM CITRATE (UNII: 1Q73Q2JULR) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49348-811-32 1 in 1 BOX 1 51 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 11/15/2006 Labeler - McKesson (177667227)