Label: MINT ANTACID 100- calcium carbonate tablet, chewable
MINT ANTACID 250- calcium carbonate tablet, chewable
- NDC Code(s): 73598-1142-1, 73598-1144-1
- Packager: JHK Inc dba American Safety & First Aid
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (In Each Tablet)
- Purpose
- Use
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Questions?
-
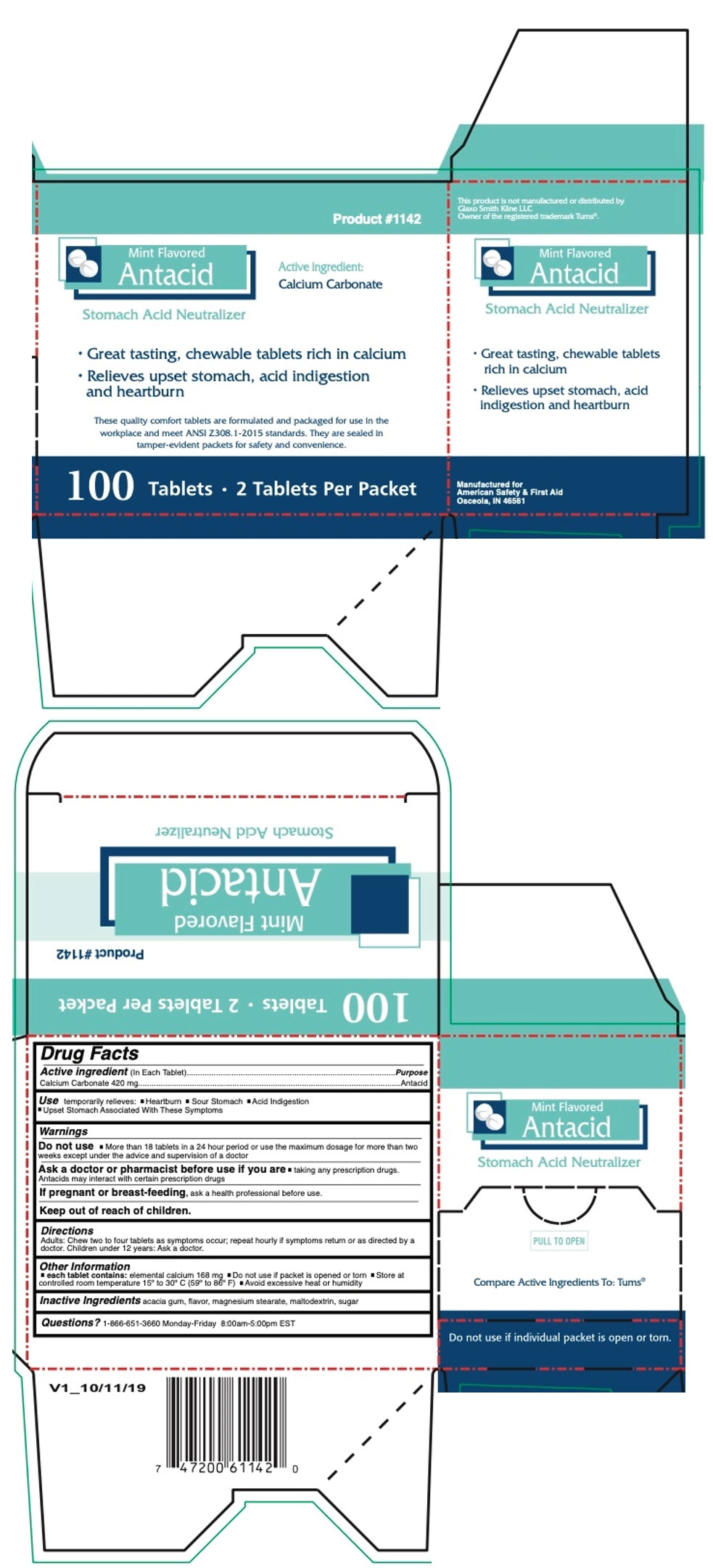
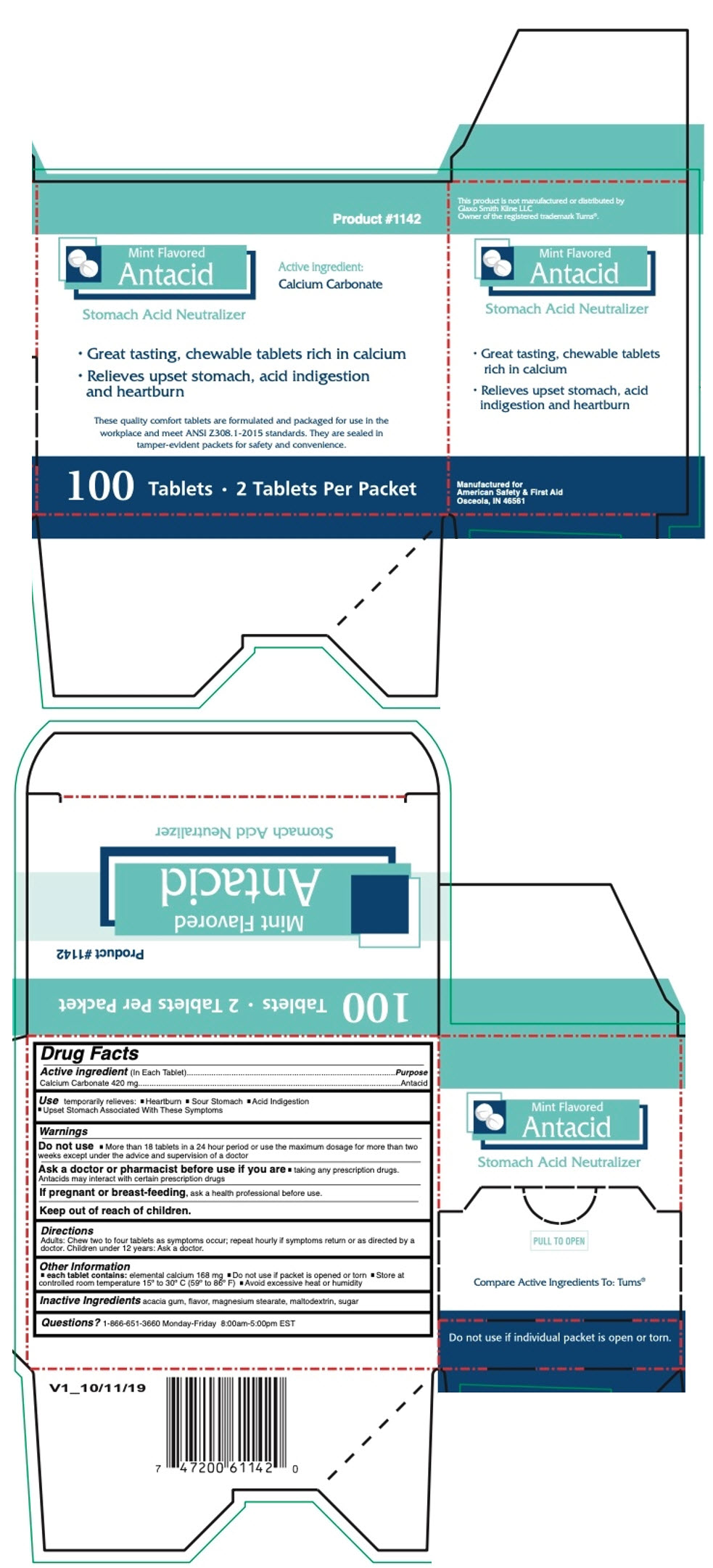
PRINCIPAL DISPLAY PANEL - 100 Tablet Box
Product #1142
Mint Flavored
AntacidActive ingredient:
Calcium CarbonateStomach Acid Neutralizer
- Great tasting, chewable tablets rich in calcium
- Relieves upset stomach, acid indigestion
and heartburn
These quality comfort tablets are formulated and packaged for use in the
workplace and meet ANSI Z308.1-2015 standards. They are sealed in
tamper-evident packets for safety and convenience.100 Tablets • 2 Tablets Per Packet
-
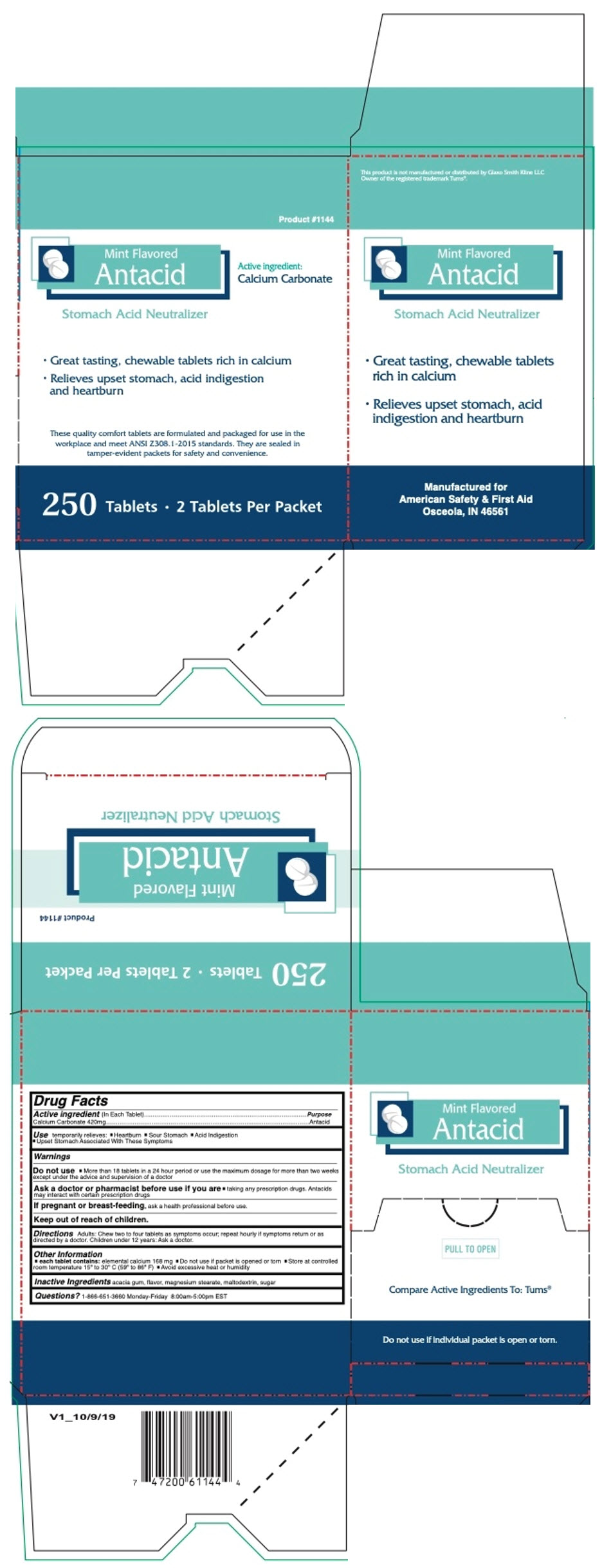
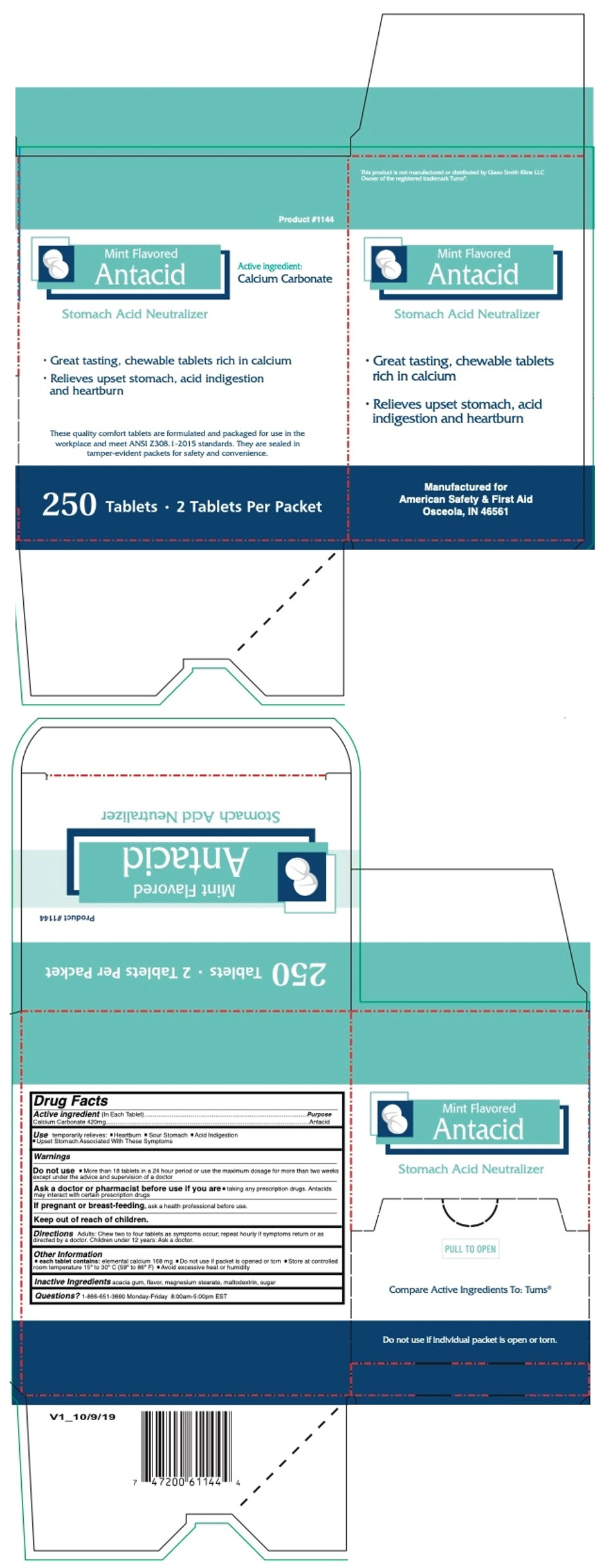
PRINCIPAL DISPLAY PANEL - 250 Tablet Box
Product #1144
Mint Flavored
AntacidActive ingredient:
Calcium CarbonateStomach Acid Neutralizer
- Great tasting, chewable tablets rich in calcium
- Relieves upset stomach, acid indigestion
and heartburn
These quality comfort tablets are formulated and packaged for use in the
workplace and meet ANSI Z308.1-2015 standards. They are sealed in
tamper-evident packets for safety and convenience.250 Tablets • 2 Tablets Per Packet
-
INGREDIENTS AND APPEARANCE
MINT ANTACID 100
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73598-1142 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 420 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) SUCROSE (UNII: C151H8M554) Product Characteristics Color WHITE Score no score Shape ROUND Size 12mm Flavor MINT Imprint Code AZ036 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73598-1142-1 50 in 1 BOX 02/02/2000 1 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M001 02/02/2000 MINT ANTACID 250
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73598-1144 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 420 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) SUCROSE (UNII: C151H8M554) Product Characteristics Color WHITE Score no score Shape ROUND Size 12mm Flavor MINT Imprint Code AZ036 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73598-1144-1 125 in 1 BOX 02/02/2000 1 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M001 02/02/2000 Labeler - JHK Inc dba American Safety & First Aid (867236309)