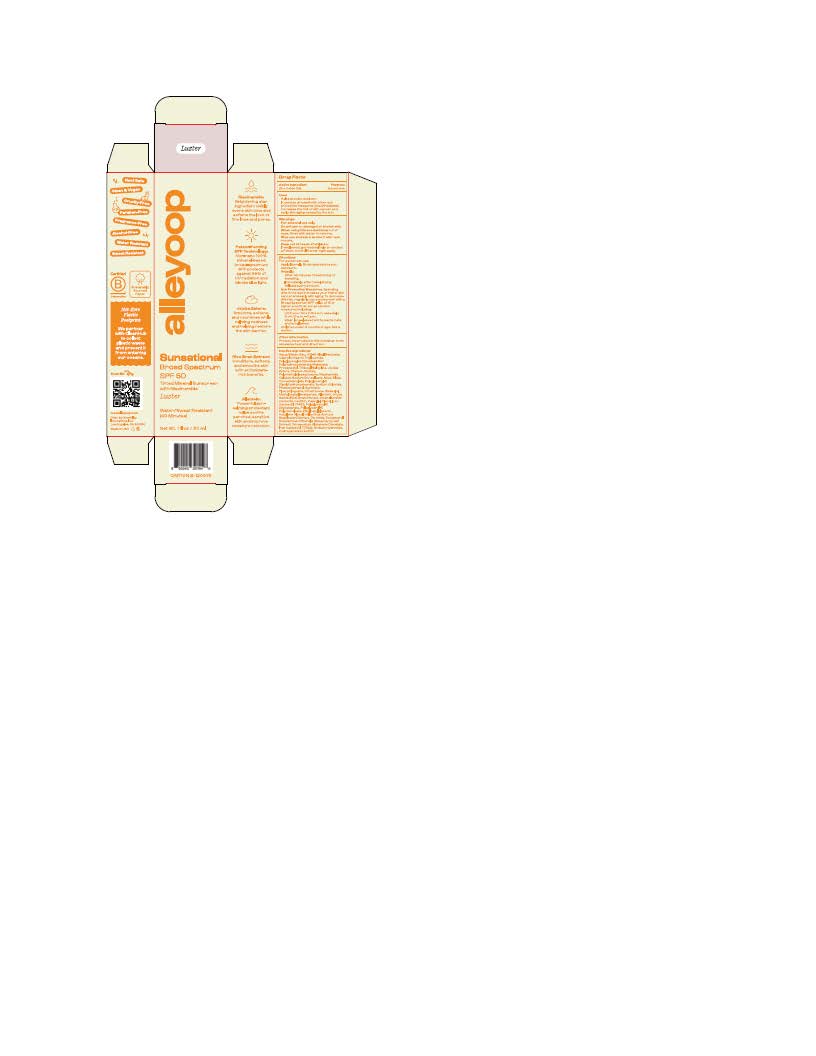
Label: ALLEYOOP SUNSATIONAL BROAD SPECTRUM- zinc oxide lotion
- NDC Code(s): 84830-2431-1, 84830-2431-2
- Packager: NewAlley Innovations Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Alleyoop Sunsational Broad Spectrum
- Alleyoop Sunsational Broad Spectrum
- Alleyoop Sunsational Broad Spectrum
- Alleyoop Sunsational Broad Spectrum
- Alleyoop Sunsational Broad Spectrum
- Alleyoop Sunsational Broad Spectrum
- Alleyoop Sunsational Broad SpectrumAlleyoop Sunsational Broad Spectrum
- Alleyoop Sunsational Broad Spectrum
-
INGREDIENTS AND APPEARANCE
ALLEYOOP SUNSATIONAL BROAD SPECTRUM
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84830-2431 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 132 mg in 1 mL Inactive Ingredients Ingredient Name Strength ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) WATER (UNII: 059QF0KO0R) NIACINAMIDE (UNII: 25X51I8RD4) PROPANEDIOL (UNII: 5965N8W85T) SODIUM CHLORIDE (UNII: 451W47IQ8X) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ROSMARINUS OFFICINALIS WHOLE (UNII: EA3289138M) METHYLHEPTYL ISOSTEARATE (UNII: 981F40Q9FF) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) ORYZA SATIVA WHOLE (UNII: 84IVV0906Z) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) COCO-CAPRYLATE (UNII: 4828G836N6) TOCOPHEROL (UNII: R0ZB2556P8) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) MICA (UNII: V8A1AW0880) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) TRIDECYL SALICYLATE (UNII: AZQ08K38Z1) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE RED (UNII: 1K09F3G675) HYDROLYZED JOJOBA ESTERS (ACID FORM) (UNII: UDR641JW8W) CALCIUM SODIUM BOROSILICATE (UNII: 4MM76N4WMY) DIMETHICONE 100 (UNII: RO266O364U) PHENOXYETHANOL (UNII: HIE492ZZ3T) HEXYLENE GLYCOL (UNII: KEH0A3F75J) HELIANTHUS ANNUUS WHOLE (UNII: 17S27ZT6KR) Product Characteristics Color pink Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84830-2431-2 1 in 1 CARTON 07/17/2024 1 NDC:84830-2431-1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/17/2024 Labeler - NewAlley Innovations Inc (119295757) Registrant - Nanophase Technologies Corporation (623502044) Establishment Name Address ID/FEI Business Operations Nanophase Technologies Corporation 050383046 api manufacture(84830-2431) Establishment Name Address ID/FEI Business Operations Nanophase Technologies Corporation 118812921 pack(84830-2431) , manufacture(84830-2431) Establishment Name Address ID/FEI Business Operations Nanophase Technologies Corporation 623502044 api manufacture(84830-2431) , manufacture(84830-2431)