Label: POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 48W- homosalate, zinc oxide lotion
POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 44N- homosalate, zinc oxide lotion
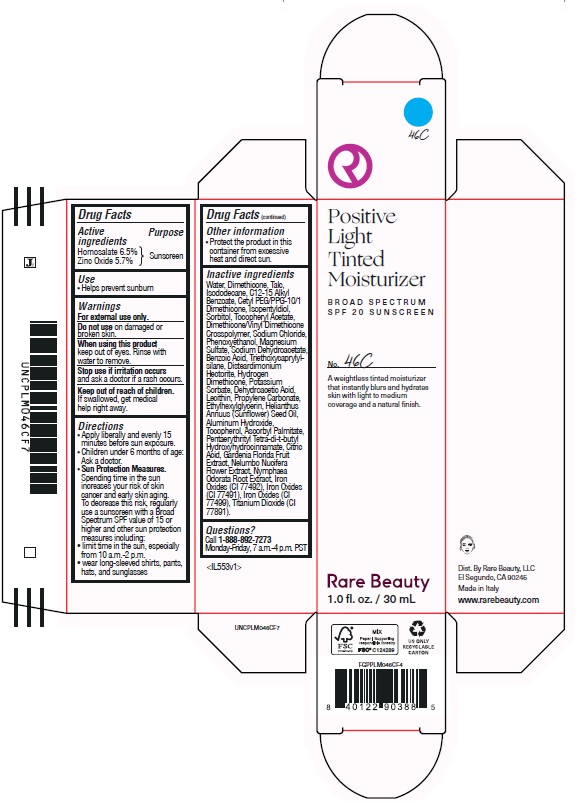
POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 46C- homosalate, zinc oxide lotion
POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 50N- homosalate, zinc oxide lotion
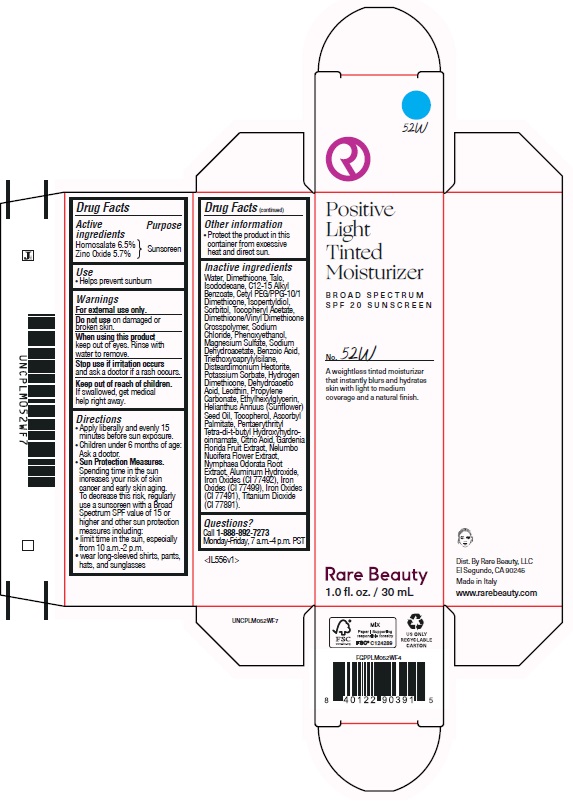
POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 52W- homosalate, zinc oxide lotion
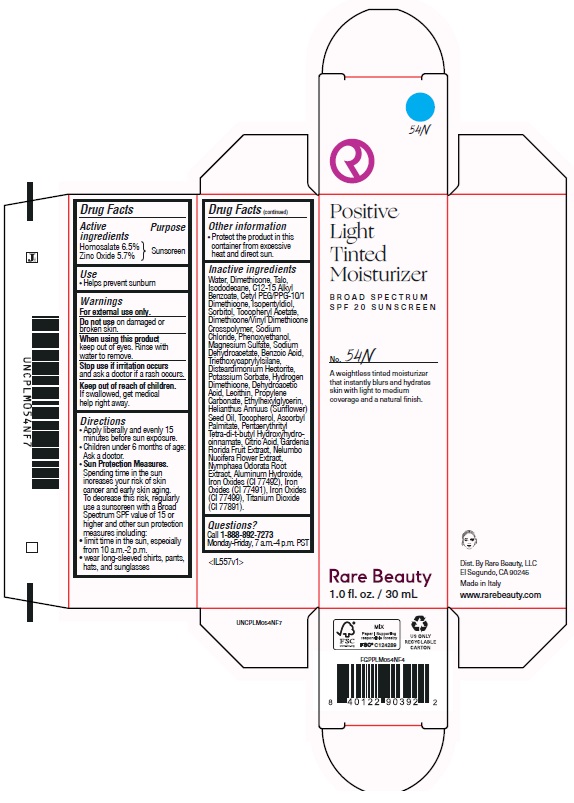
POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 54N- homosalate, zinc oxide lotion
POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 56N- homosalate, zinc oxide lotion
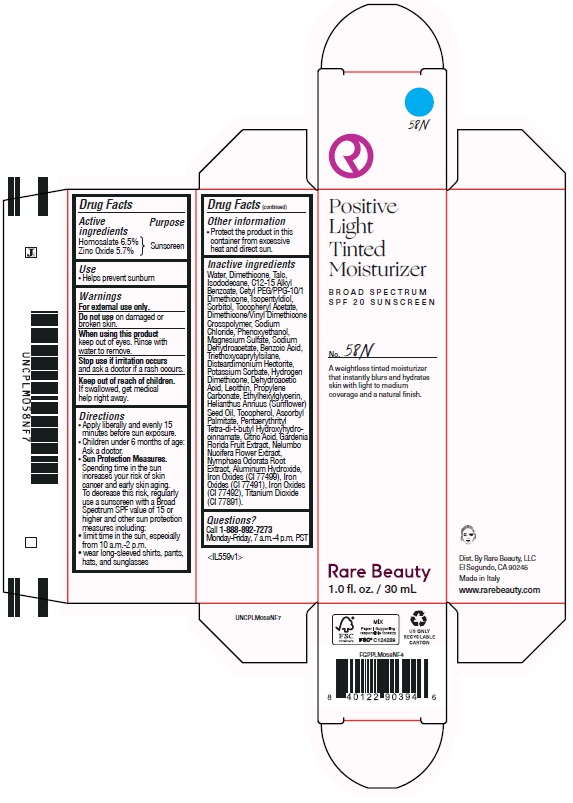
POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 58N- homosalate, zinc oxide lotion
-
NDC Code(s):
82109-140-01,
82109-141-01,
82109-142-01,
82109-143-01, view more82109-144-01, 82109-145-01, 82109-146-01, 82109-147-01
- Packager: Rare Beauty, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
-
Directions
- Apply liberally and evenly 15 minutes before sun exposure.
- Children under 6 months of age: Ask a doctor.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Other Information
-
Inactive Ingredients
Water, Dimethicone, Talc, Isododecane, C12-15 Alkyl Benzoate, Cetyl PEG/PPG-10/1 Dimethicone, Isopentyldiol, Sorbitol, Tocopheryl Acetate, Dimethicone/Vinyl Dimethicone Crosspolymer, Sodium Chloride, Phenoxyethanol, Magnesium Sulfate, Sodium Dehydroacetate, Benzoic Acid, Hydrogen Dimethicone, Triethoxycaprylylsilane, Disteardimonium Hectorite, Potassium Sorbate, Aluminum Hydroxide, Dehydroacetic Acid, Lecithin, Propylene Carbonate, Ethylhexylglycerin, Helianthus Annuus (Sunflower) Seed Oil, Tocopherol, Ascorbyl Palmitate, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, Citric Acid, Gardenia Florida Fruit Extract, Nelumbo Nucifera Flower Extract, Nymphaea Odorata Root Extract, Titanium Dioxide (CI 77891), Iron Oxides (CI 77492), Iron Oxides (CI 77491), Iron Oxides (CI 77499).
- Questions?
- Company Information
- 44N
- 46C
- 48W
- 50N
- 52W
- 54N
- 56N
- 58N
-
INGREDIENTS AND APPEARANCE
POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 48W
homosalate, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82109-142 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 65 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 57 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPENTYLDIOL (UNII: 19NOL5474Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROGEN DIMETHICONE (20 CST) (UNII: 12Z59IF64N) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) BENZOIC ACID (UNII: 8SKN0B0MIM) WATER (UNII: 059QF0KO0R) DEHYDROACETIC ACID (UNII: 2KAG279R6R) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ASCORBYL PALMITATE (UNII: QN83US2B0N) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GARDENIA JASMINOIDES FRUIT (UNII: 7CTH8MD549) NYMPHAEA ODORATA ROOT (UNII: 69RAZ4C432) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) ISODODECANE (UNII: A8289P68Y2) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) SUNFLOWER OIL (UNII: 3W1JG795YI) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SORBITOL (UNII: 506T60A25R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82109-142-01 1 in 1 CARTON 11/01/2024 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2024 POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 44N
homosalate, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82109-140 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 65 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 57 mg in 1 mL Inactive Ingredients Ingredient Name Strength PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SORBITOL (UNII: 506T60A25R) ISOPENTYLDIOL (UNII: 19NOL5474Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROGEN DIMETHICONE (20 CST) (UNII: 12Z59IF64N) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) BENZOIC ACID (UNII: 8SKN0B0MIM) WATER (UNII: 059QF0KO0R) DEHYDROACETIC ACID (UNII: 2KAG279R6R) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ASCORBYL PALMITATE (UNII: QN83US2B0N) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GARDENIA JASMINOIDES FRUIT (UNII: 7CTH8MD549) NYMPHAEA ODORATA ROOT (UNII: 69RAZ4C432) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) ISODODECANE (UNII: A8289P68Y2) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) SUNFLOWER OIL (UNII: 3W1JG795YI) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82109-140-01 1 in 1 CARTON 11/01/2024 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2024 POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 46C
homosalate, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82109-141 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 65 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 57 mg in 1 mL Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) SUNFLOWER OIL (UNII: 3W1JG795YI) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SORBITOL (UNII: 506T60A25R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ISOPENTYLDIOL (UNII: 19NOL5474Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROGEN DIMETHICONE (20 CST) (UNII: 12Z59IF64N) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) BENZOIC ACID (UNII: 8SKN0B0MIM) WATER (UNII: 059QF0KO0R) DEHYDROACETIC ACID (UNII: 2KAG279R6R) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ASCORBYL PALMITATE (UNII: QN83US2B0N) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GARDENIA JASMINOIDES FRUIT (UNII: 7CTH8MD549) NYMPHAEA ODORATA ROOT (UNII: 69RAZ4C432) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) ISODODECANE (UNII: A8289P68Y2) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82109-141-01 1 in 1 CARTON 11/01/2024 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2024 POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 50N
homosalate, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82109-143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 65 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 57 mg in 1 mL Inactive Ingredients Ingredient Name Strength PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SORBITOL (UNII: 506T60A25R) ISOPENTYLDIOL (UNII: 19NOL5474Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) SODIUM CHLORIDE (UNII: 451W47IQ8X) SUNFLOWER OIL (UNII: 3W1JG795YI) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (20 CST) (UNII: 12Z59IF64N) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) BENZOIC ACID (UNII: 8SKN0B0MIM) WATER (UNII: 059QF0KO0R) DEHYDROACETIC ACID (UNII: 2KAG279R6R) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ASCORBYL PALMITATE (UNII: QN83US2B0N) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GARDENIA JASMINOIDES FRUIT (UNII: 7CTH8MD549) NYMPHAEA ODORATA ROOT (UNII: 69RAZ4C432) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) ISODODECANE (UNII: A8289P68Y2) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82109-143-01 1 in 1 CARTON 11/01/2024 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2024 POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 52W
homosalate, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82109-144 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 65 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 57 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPENTYLDIOL (UNII: 19NOL5474Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROGEN DIMETHICONE (20 CST) (UNII: 12Z59IF64N) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) BENZOIC ACID (UNII: 8SKN0B0MIM) WATER (UNII: 059QF0KO0R) DEHYDROACETIC ACID (UNII: 2KAG279R6R) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ASCORBYL PALMITATE (UNII: QN83US2B0N) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GARDENIA JASMINOIDES FRUIT (UNII: 7CTH8MD549) NYMPHAEA ODORATA ROOT (UNII: 69RAZ4C432) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) ISODODECANE (UNII: A8289P68Y2) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) SUNFLOWER OIL (UNII: 3W1JG795YI) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SORBITOL (UNII: 506T60A25R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82109-144-01 1 in 1 CARTON 11/01/2024 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2024 POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 54N
homosalate, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82109-145 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 65 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 57 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) SUNFLOWER OIL (UNII: 3W1JG795YI) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SORBITOL (UNII: 506T60A25R) ISOPENTYLDIOL (UNII: 19NOL5474Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROGEN DIMETHICONE (20 CST) (UNII: 12Z59IF64N) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) BENZOIC ACID (UNII: 8SKN0B0MIM) WATER (UNII: 059QF0KO0R) DEHYDROACETIC ACID (UNII: 2KAG279R6R) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ASCORBYL PALMITATE (UNII: QN83US2B0N) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GARDENIA JASMINOIDES FRUIT (UNII: 7CTH8MD549) NYMPHAEA ODORATA ROOT (UNII: 69RAZ4C432) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) ISODODECANE (UNII: A8289P68Y2) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82109-145-01 1 in 1 CARTON 11/01/2024 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2024 POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 56N
homosalate, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82109-146 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 65 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 57 mg in 1 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ASCORBYL PALMITATE (UNII: QN83US2B0N) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GARDENIA JASMINOIDES FRUIT (UNII: 7CTH8MD549) NYMPHAEA ODORATA ROOT (UNII: 69RAZ4C432) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) ISODODECANE (UNII: A8289P68Y2) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) SUNFLOWER OIL (UNII: 3W1JG795YI) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SORBITOL (UNII: 506T60A25R) ISOPENTYLDIOL (UNII: 19NOL5474Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROGEN DIMETHICONE (20 CST) (UNII: 12Z59IF64N) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) BENZOIC ACID (UNII: 8SKN0B0MIM) WATER (UNII: 059QF0KO0R) DEHYDROACETIC ACID (UNII: 2KAG279R6R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82109-146-01 1 in 1 CARTON 11/01/2024 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2024 POSITIVE LIGHT TINTED MOISTURIZER BROAD SPECTRUM SPF 20 SUNSCREEN 58N
homosalate, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82109-147 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 65 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 57 mg in 1 mL Inactive Ingredients Ingredient Name Strength PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SORBITOL (UNII: 506T60A25R) ISOPENTYLDIOL (UNII: 19NOL5474Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) SODIUM CHLORIDE (UNII: 451W47IQ8X) SUNFLOWER OIL (UNII: 3W1JG795YI) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (20 CST) (UNII: 12Z59IF64N) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) BENZOIC ACID (UNII: 8SKN0B0MIM) DEHYDROACETIC ACID (UNII: 2KAG279R6R) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ASCORBYL PALMITATE (UNII: QN83US2B0N) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GARDENIA JASMINOIDES FRUIT (UNII: 7CTH8MD549) NYMPHAEA ODORATA ROOT (UNII: 69RAZ4C432) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) ISODODECANE (UNII: A8289P68Y2) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82109-147-01 1 in 1 CARTON 11/01/2024 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2024 Labeler - Rare Beauty, LLC (117041437)