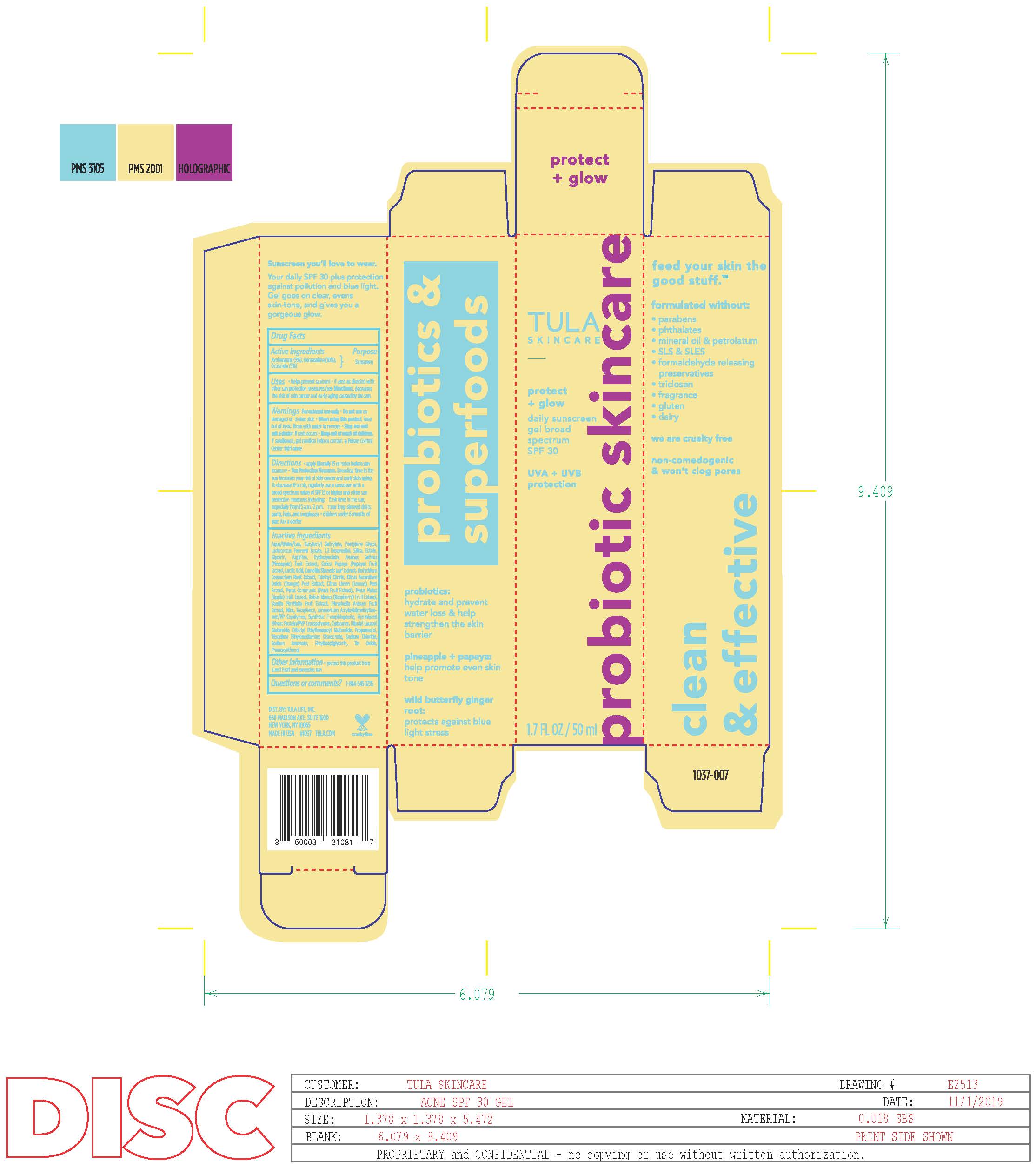
Label: TULA SKINCARE PROTECT GLOW DAILY SUNSCREEN GEL BROAD SPECTRUM SPF 30 lotion
- NDC Code(s): 72296-050-50
- Packager: Tula Life LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warning
- Keep out of reach of children.
-
Directions
• apply generously 15 minutes before sun exposure
• reapply at least every 2 hours
• use a water-resistant sunscreen if swimming or sweating
• Sun Protection Measures. Spending time in the sun
increases your risk of skin cancer and early skin aging. To decrease
this risk, regularly use a sunscreen with a Broad Spectrum SPF
value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. - 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
• children under 6 months of age: Ask a doctor -
Inactive ingredients
Aqua/Water/Eau, Butyloctyl Salicylate, Pentylene Glycol,
Lactococcus Ferment Lysate, 1,2-Hexanediol, Silica, Ectoin,
Glycerin, Arginine, Hydroxyectoin, Ananas Sativus
(Pineapple) Fruit Extract, Carica Papaya (Papaya) Fruit
Extract, Lactic Acid, Camellia Sinensis Leaf Extract, Hedychium
Coronarium Root Extract, Triethyl Citrate, Citrus Aurantium
Dulcis (Orange) Peel Extract, Citrus Limon (Lemon) Peel
Extract, Pyrus Communis (Pear) Fruit Extract, Pyrus Malus
(Apple) Fruit Extract, Rubus Idaeus (Raspberry) Fruit Extract,
Vanilla Planifolia Fruit Extract, Pimpinella Anisum Fruit
Extract, Mica, Tocopherol, Ammonium Acryloyldimethyltaurate/
VP Copolymer, Synthetic Fluorphlogopite, Hydrolyzed
Wheat Protein/PVP Crosspolymer, Carbomer, Dibutyl Lauroyl
Glutamide, Dibutyl Ethylhexanoyl Glutamide, Propanediol,
Trisodium Ethylenediamine Disuccinate, Sodium Chloride,
Sodium Benzoate, Ehtylhexylglycerin, Tin Oxide,
Phenoxyethanol - Other Information
- Questions or Comments?
- Principal Dispplay Panel
-
INGREDIENTS AND APPEARANCE
TULA SKINCARE PROTECT GLOW DAILY SUNSCREEN GEL BROAD SPECTRUM SPF 30
tula skincare protect glow daily sunscreen gel broad spectrum spf 30 lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72296-050 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTOCOCCUS LACTIS (UNII: F1A0PSN10V) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ECTOINE (UNII: 7GXZ3858RY) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TOCOPHEROL (UNII: R0ZB2556P8) SILICON (UNII: Z4152N8IUI) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) HYDROLYZED WHEAT PROTEIN (ENZYMATIC, 3000 MW) (UNII: J2S07SB0YL) ARGININE (UNII: 94ZLA3W45F) CARBOMER 940 (UNII: 4Q93RCW27E) DIBUTYL LAUROYL GLUTAMIDE (UNII: 3V7K3IA58X) DIBUTYL ETHYLHEXANOYL GLUTAMIDE (UNII: 0IAF2L30VS) HYDROXYECTOIN (UNII: CIJ7YN252E) PINEAPPLE (UNII: 2A88ZO081O) CARICA PAPAYA LEAF (UNII: 66J7636Z2I) PROPANEDIOL (UNII: 5965N8W85T) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) LACTIC ACID (UNII: 33X04XA5AT) DISODIUM EDTA-COPPER (UNII: 6V475AX06U) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM BENZOATE (UNII: OJ245FE5EU) CAMELLIA SINENSIS FLOWER (UNII: 9I2BJY2J17) HEDYCHIUM CORONARIUM ROOT (UNII: 92A6N0IQN9) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CITRUS AURANTIUM FRUIT (UNII: DQD16J2B5O) PEAR (UNII: 2ZN8DWC0YF) RUBUS IDAEUS LEAF (UNII: 8O2V33JG64) VANILLA PLANIFOLIA LEAF (UNII: WER36ZQQ2R) PIMPINELLA ANISUM WHOLE (UNII: HO63CL229O) APPLE (UNII: B423VGH5S9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72296-050-50 1 in 1 CARTON 03/22/2024 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/22/2024 Labeler - Tula Life LLC (080051358)