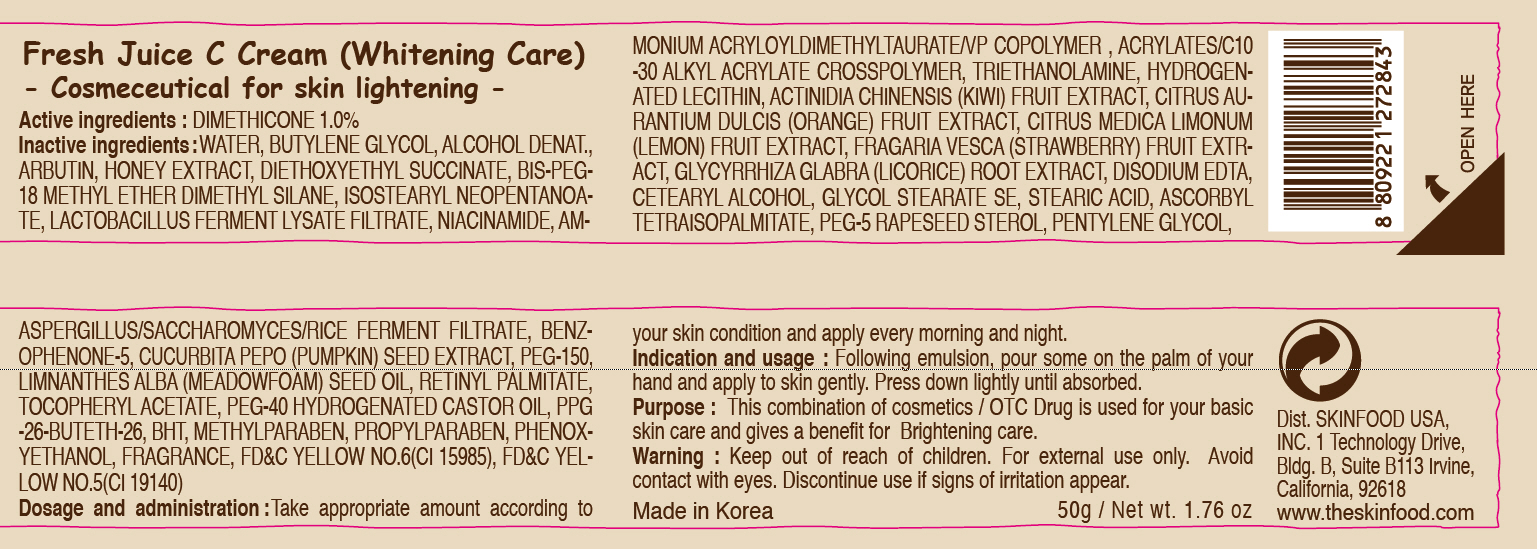
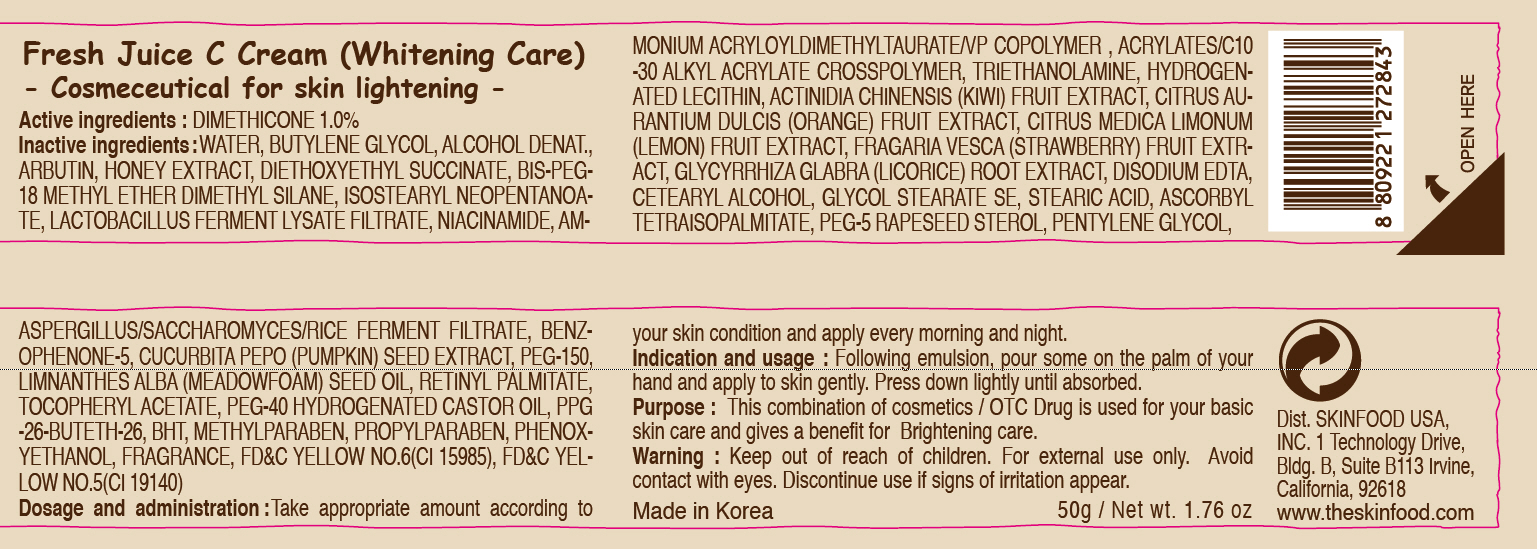
Label: FRESH JUICE C WHITENING CARE- dimethicone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 76214-023-01 - Packager: SKINFOOD CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 22, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive ingredients:
WATER, BUTYLENE GLYCOL, ALCOHOL DENAT., ARBUTIN, HONEY EXTRACT, DIETHOXYETHYL SUCCINATE, BIS-PEG-18 METHYL ETHER DIMETHYL SILANE, ISOSTEARYL NEOPENTANOATE, LACTOBACILLUS FERMENT LYSATE FILTRATE, NIACINAMIDE, AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER , ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, TRIETHANOLAMINE, HYDROGENATED LECITHIN, ACTINIDIA CHINENSIS (KIWI) FRUIT EXTRACT, CITRUS AURANTIUM DULCIS (ORANGE) FRUIT EXTRACT, CITRUS MEDICA LIMONUM (LEMON) FRUIT EXTRACT, FRAGARIA VESCA (STRAWBERRY) FRUIT EXTRACT, GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT, DISODIUM EDTA, CETEARYL ALCOHOL, GLYCOL STEARATE SE, STEARIC ACID, ASCORBYL TETRAISOPALMITATE, PEG-5 RAPESEED STEROL, PENTYLENE GLYCOL, ASPERGILLUS/SACCHAROMYCES/RICE FERMENT FILTRATE, BENZOPHENONE-5, CUCURBITA PEPO (PUMPKIN) SEED EXTRACT, PEG-150, LIMNANTHES ALBA (MEADOWFOAM) SEED OIL, RETINYL PALMITATE, TOCOPHERYL ACETATE, PEG-40 HYDROGENATED CASTOR OIL, PPG-26-BUTETH-26, BHT, METHYLPARABEN, PROPYLPARABEN, PHENOXYETHANOL, FRAGRANCE - PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FRESH JUICE C WHITENING CARE
dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76214-023 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 0.5 g in 50 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ARBUTIN (UNII: C5INA23HXF) HONEY (UNII: Y9H1V576FH) NIACINAMIDE (UNII: 25X51I8RD4) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) TROLAMINE (UNII: 9O3K93S3TK) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) KIWI FRUIT (UNII: 71ES77LGJC) ORANGE (UNII: 5EVU04N5QU) LEMON (UNII: 24RS0A988O) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) EDETATE DISODIUM (UNII: 7FLD91C86K) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) STEARIC ACID (UNII: 4ELV7Z65AP) POLYETHYLENE GLYCOL 7000 (UNII: Q0JET65GEL) PENTYLENE GLYCOL (UNII: 50C1307PZG) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PUMPKIN SEED (UNII: GH30P1VXK2) PROPYLPARABEN (UNII: Z8IX2SC1OH) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76214-023-01 50 g in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 03/01/2011 Labeler - SKINFOOD CO., LTD. (690324173) Registrant - SKINFOOD CO., LTD. (690324173) Establishment Name Address ID/FEI Business Operations SKINFOOD CO., LTD. 690324173 manufacture