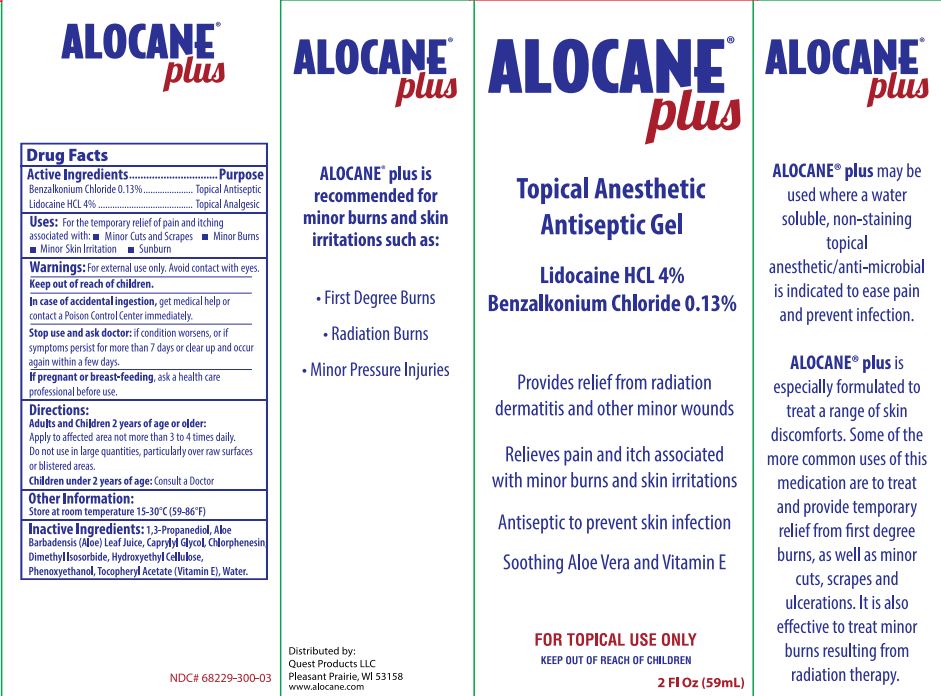
Label: ALOCANE PLUS- lidocaine hydrochloride and benzalkonium chloride gel
- NDC Code(s): 68229-300-01, 68229-300-02, 68229-300-03, 68229-300-04
- Packager: Quest Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 59 mL Tube Carton
- 946mL Bottle
-
INGREDIENTS AND APPEARANCE
ALOCANE PLUS
lidocaine hydrochloride and benzalkonium chloride gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68229-300 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 g in 100 mL BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength PROPANEDIOL (UNII: 5965N8W85T) ALOE VERA LEAF (UNII: ZY81Z83H0X) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) HYDROXYETHYL CELLULOSE (5500 MPA.S AT 2%) (UNII: M825OX60H9) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68229-300-02 50 in 1 BOX 12/11/2013 07/12/2021 1 NDC:68229-300-01 3.55 mL in 1 DOSE PACK; Type 0: Not a Combination Product 2 NDC:68229-300-03 1 in 1 CARTON 03/01/2020 2 59 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:68229-300-04 946 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/09/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/11/2013 Labeler - Quest Products, Inc. (075402441) Establishment Name Address ID/FEI Business Operations Fill Tech USA 926433855 manufacture(68229-300)