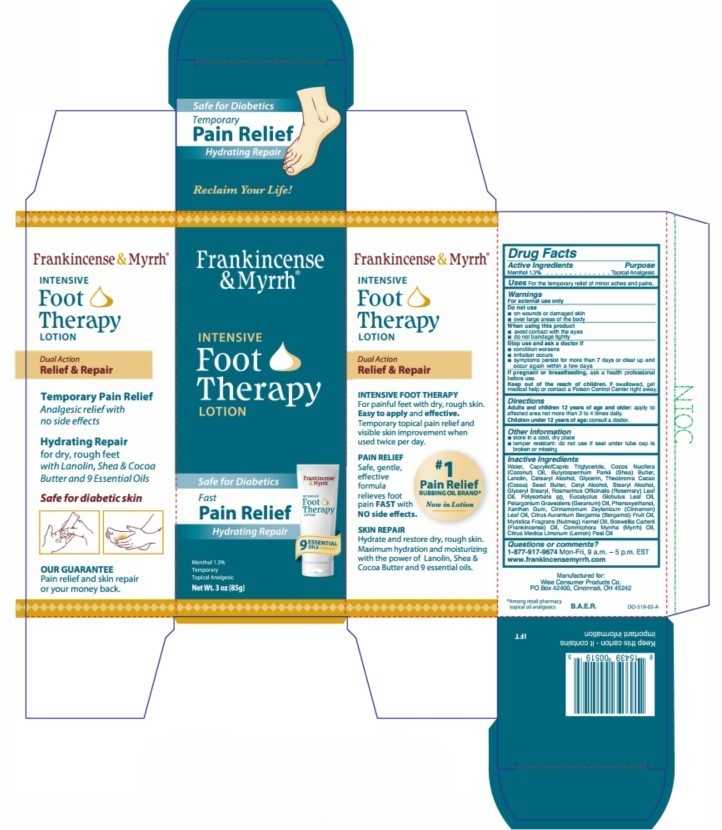
Label: FRANKINCENSE AND MYRRH INTENSIVE FOOT THERAPY- menthol lotion
- NDC Code(s): 42346-520-85
- Packager: Wise Consumer Products
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water, Caprylic/Capric Triglyceride, Cocos Nucifera (Coconut) Oil, Butyrospermum Parkii (Shea) Butter, Lanolin, Cetearyl Alcohol, Glycerin, Theobroma Cacao (Cocoa) Seed Butter, Cetyl Alcohol, Stearyl Alcohol, Glyceryl Stearyl, Rosmarinus Officinalis (Rosemary) Leaf Oil, Polysorbate 60, Eucalyptus Globulus Leaf Oil, Pelargonium Graveolens (Geranium) Oil, Phenoxyethanol, Xanthan Gum, Cinnamomum Zeylanicum (Cinnamon) Leaf Oil, Citrus Aurantium Bergamia (Bergamot) Fruit Oil, Myristica Fragrans (Nutmeg) Kernel Oil, Boswellia Carterii (Frankincense) Oil, Commiphora Myrrha (Myrrh) Oil, Citrus Medica Limonum (Lemon) Peel Oil
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FRANKINCENSE AND MYRRH INTENSIVE FOOT THERAPY
menthol lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42346-520 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1.3 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GERANIUM OIL, ALGERIAN TYPE (UNII: 5Q1I94P4WG) NUTMEG OIL (UNII: Z1CLM48948) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) POLYSORBATE 60 (UNII: CAL22UVI4M) EUCALYPTUS OIL (UNII: 2R04ONI662) PHENOXYETHANOL (UNII: HIE492ZZ3T) MYRRH OIL (UNII: H74221J5J4) LANOLIN (UNII: 7EV65EAW6H) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) COCOA BUTTER (UNII: 512OYT1CRR) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) COCONUT OIL (UNII: Q9L0O73W7L) BERGAMOT OIL (UNII: 39W1PKE3JI) CETYL ALCOHOL (UNII: 936JST6JCN) ROSEMARY OIL (UNII: 8LGU7VM393) XANTHAN GUM (UNII: TTV12P4NEE) CINNAMON OIL (UNII: E5GY4I6YCZ) FRANKINCENSE OIL (UNII: 67ZYA5T02K) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) SHEA BUTTER (UNII: K49155WL9Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42346-520-85 1 in 1 CARTON 10/09/2024 1 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/09/2024 Labeler - Wise Consumer Products (006459643) Establishment Name Address ID/FEI Business Operations Innovation Labs, Inc. 117109069 manufacture(42346-520)