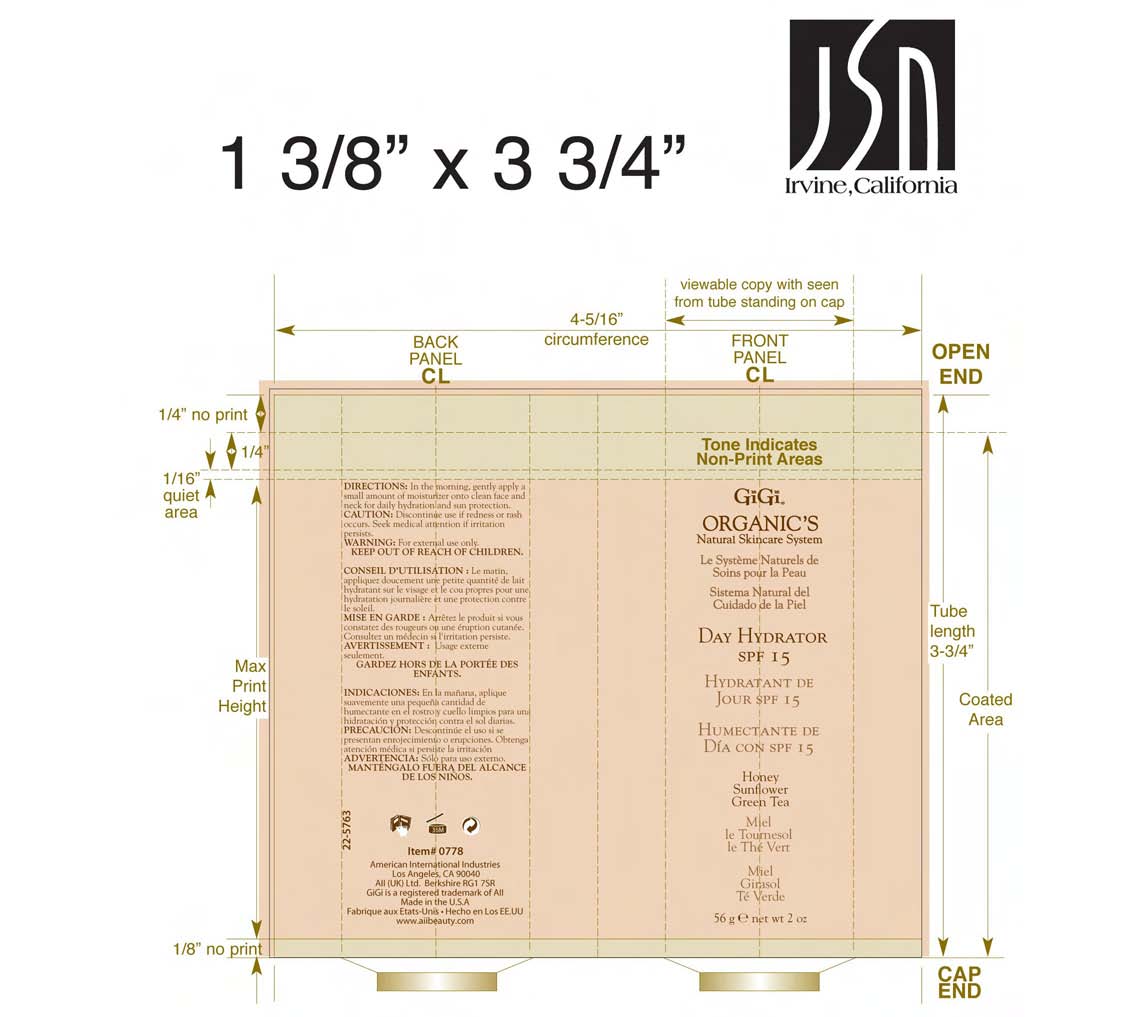
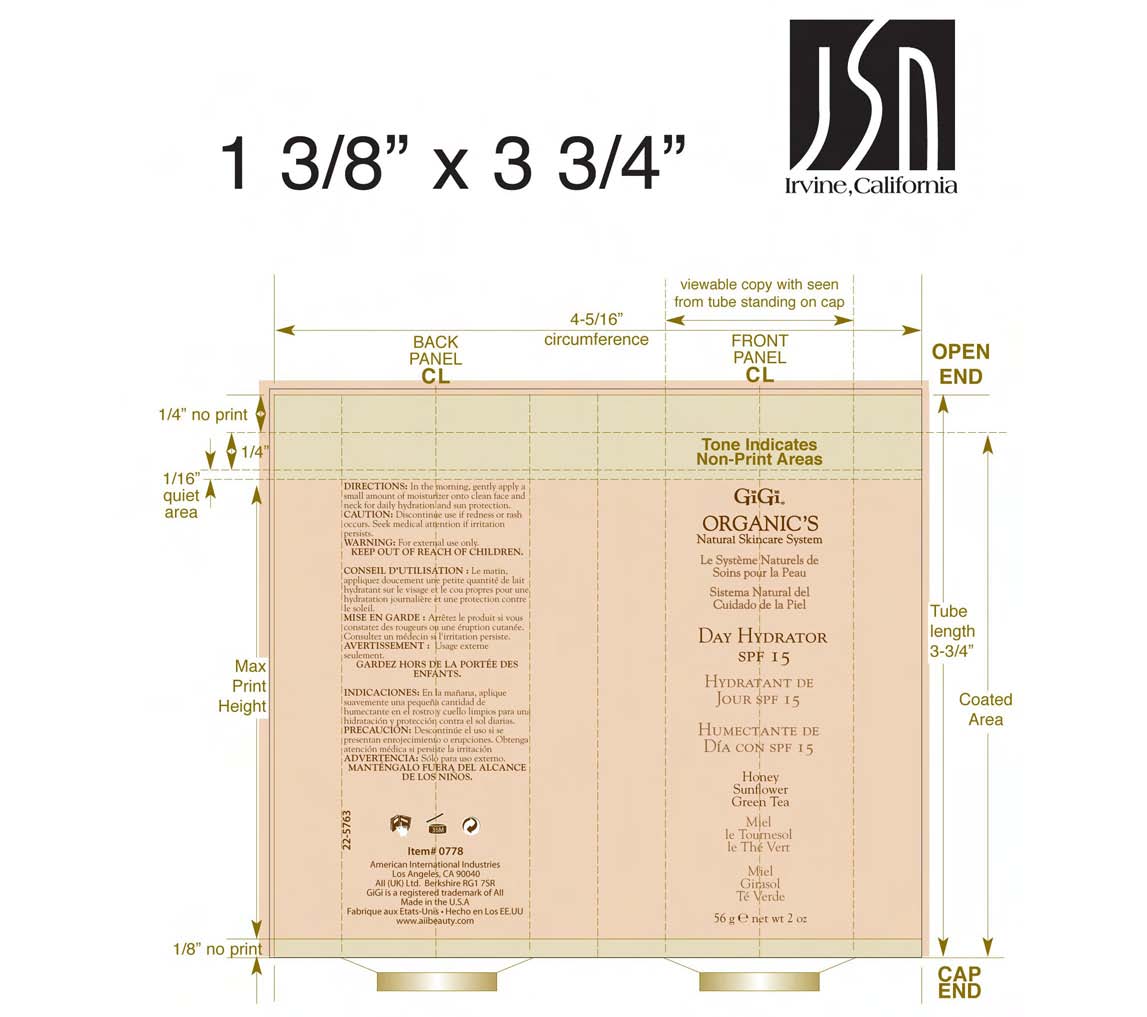
Label: DAY HYDRATOR SPF-15- octinoxate and octisalate and oxybenzone lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 57367-027-18, 57367-027-19 - Packager: 220 Laboratories Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 10, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- GENERAL PRECAUTIONS
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DAY HYDRATOR SPF-15
octinoxate and octisalate and oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57367-027 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 30 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ETHYLHEXYL PALMITATE (UNII: 2865993309) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 100 STEARATE (UNII: YD01N1999R) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) HEXYLENE GLYCOL (UNII: KEH0A3F75J) TROLAMINE (UNII: 9O3K93S3TK) MYRISTYL LAURATE (UNII: 58U0NZN2BT) ALLANTOIN (UNII: 344S277G0Z) EDETATE SODIUM (UNII: MP1J8420LU) HELIANTHUS ANNUUS (UNII: BKJ0J3D1BP) ORANGE OIL (UNII: AKN3KSD11B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PANTHENOL (UNII: WV9CM0O67Z) HONEY (UNII: Y9H1V576FH) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) BELLIS PERENNIS (UNII: 2HU33I03UY) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HIBISCUS SABDARIFFA CALYX (UNII: 584J2XT260) JASMINE (UNII: 0Q8K841432) LAVENDER (UNII: 9YT4B71U8P) ROSA CANINA FRUIT (UNII: 3TNW8D08V3) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57367-027-19 1 in 1 CARTON 1 NDC:57367-027-18 56 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/01/2006 Labeler - 220 Laboratories Inc. (783247950) Registrant - 220 Laboratories Inc. (783247950) Establishment Name Address ID/FEI Business Operations 220 Laboratories Inc. 783247950 manufacture